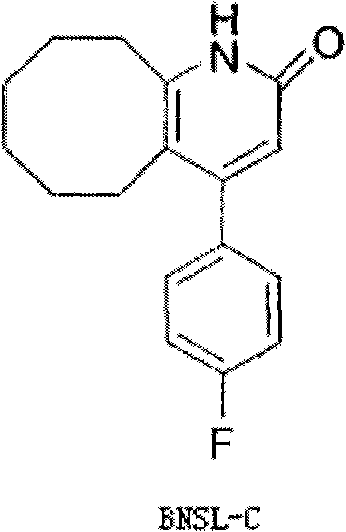
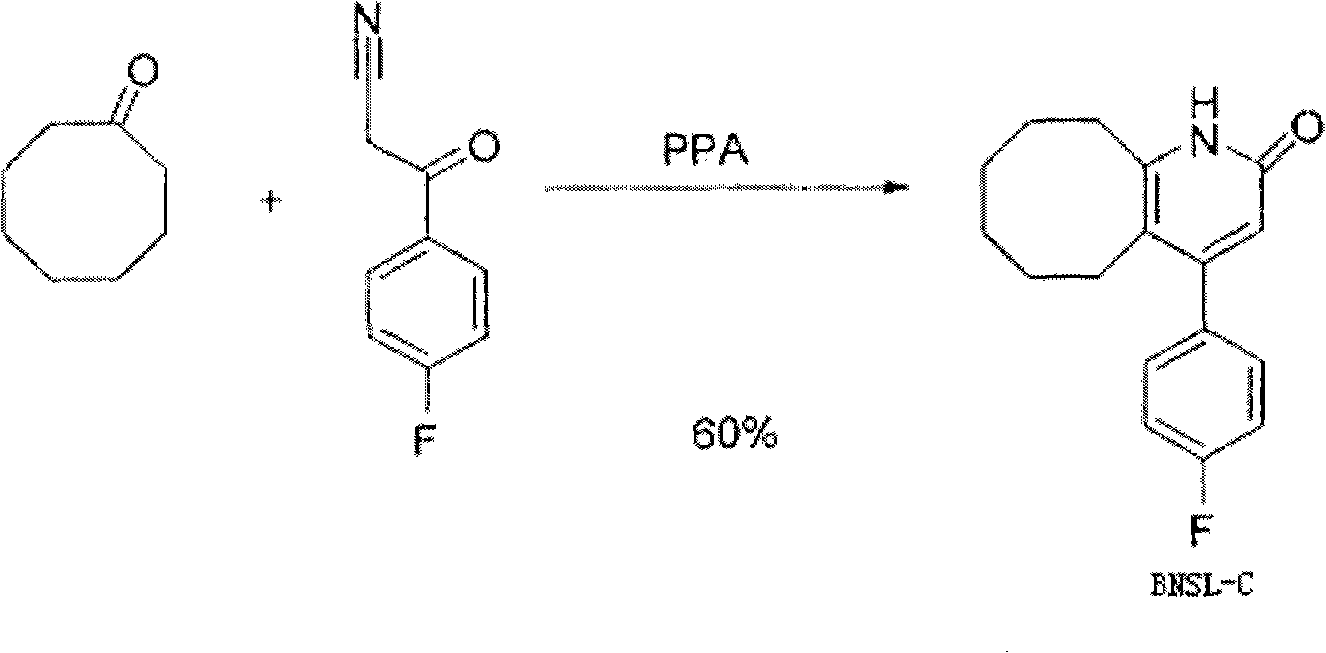
Method for preparing Blonanserin intermediate
A technology of blonanserin and intermediates, applied in the field of medicinal chemistry, can solve problems such as difficulty in separation, decreased yield, bumping, etc., and achieve the effects of stable yield, shortened reaction time, and reduced usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of BNSL-A【3-(4-fluorophenyl)-3-oxopropionitrile】
[0028] Add 480 ml of toluene to a 1000 ml four-necked flask equipped with a mechanical stirrer, a thermometer, a reflux condenser, a drying tube, and a dropping funnel. Add 40.8 g (1.02 mol) of sodium hydride (60% content) and 39.6 g (0.97 mol) of acetonitrile into the reaction flask under stirring. A toluene solution of methyl p-fluorobenzoate [75.0 g (0.49 mol) methyl p-fluorobenzoate dissolved in 72 ml of toluene] was added dropwise. After the dropwise addition was completed, 39.6 g (0.97 mol) of acetonitrile was added after heating to 90° C. for 2 hours, and then reacted at 90° C. for another 5 hours. TLC (Thin Layer Chromatography, Thin Layer Chromatography) monitored the completion of the reaction, cooled, and suction filtered to obtain a yellow sodium salt. Sodium salt was dissolved in 840 ml of water, and a small amount of toluene in the upper layer was removed. The solution was orange-red and tr...
Embodiment 2
[0044] (1) Synthesis of BNSL-A【3-(4-fluorophenyl)-3-oxopropionitrile】
[0045] Add 900 milliliters of toluene to a 2000 milliliter four-necked flask equipped with a mechanical stirrer, a thermometer, a reflux condenser, a drying tube, and a dropping funnel. Add 87.7 g (2.19 mol) of sodium hydride (60% content) and 82.9 g (2.02 mol) of acetonitrile into the reaction flask under stirring. A toluene solution of methyl p-fluorobenzoate [155.6 g (1.01 mol) methyl p-fluorobenzoate dissolved in 150 ml of toluene] was added dropwise. After the dropwise addition was completed, 82.9 g (2.02 mol) of acetonitrile was added after heating to 90° C. for 2 hours, and then reacted at 90° C. for another 5 hours. The completion of the reaction was monitored by TLC, cooled, and suction filtered to obtain a yellow sodium salt. Sodium salt was dissolved in 1680 ml of water, a small amount of toluene in the upper layer was removed, 695 ml of 3M hydrochloric acid was added dropwise with stirring, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com