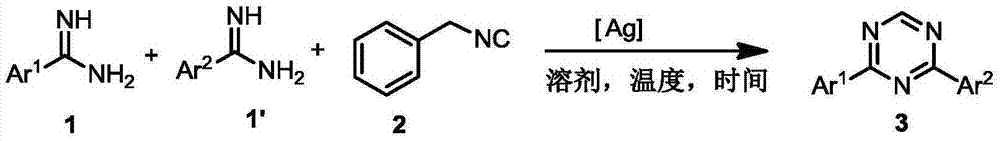
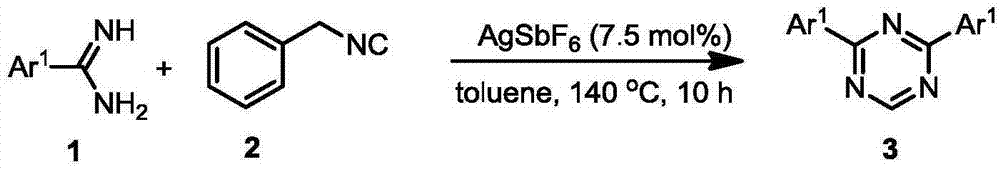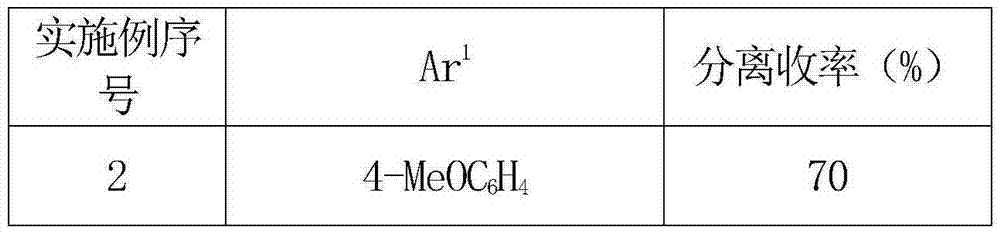Preparation method for 2,4-disubstituted-1,3,5-triazine derivatives
A technology of triazine derivatives and disubstitution, applied in the direction of organic chemistry, can solve the problems of low yield and harsh reaction conditions, and achieve the effect of simple reaction system, easy synthesis and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In order to better understand the present invention, the synthesis of symmetric 2,4-disubstituted-1,3,5 triazine derivatives is illustrated by the following examples.
[0016]
[0017] In the reaction tube, add AgSbF in an argon atmosphere 6 (0.015mmol), amidine (1) (Ar 1 Is phenyl 0.4mmol) and benzyl isonitrile (2) (0.3mmol), then add the solvent toluene (3mL), stir under heating at 140℃ for about 10 hours, stop the reaction, cool to room temperature, transfer the reaction solution into the In the steaming flask, the solvent was removed by distillation under reduced pressure, the residue was dissolved in dichloromethane and loaded, using 300-400 mesh silica gel as the stationary phase, and the mixed solution of petroleum ether: ethyl acetate = 50:1 (volume ratio) The eluent is subjected to silica gel column chromatography, nuclear magnetic and liquid mass spectrometry detection, and 2,4-disubstituted-1,3,5 triazine (Ar 1 Is phenyl) consistent.
[0018] The following table s...
Embodiment 9
[0022] In order to better understand the present invention, the following examples illustrate the synthesis of asymmetric 2,4-disubstituted-1,3,5 triazine derivatives.
[0023]
[0024] In the reaction tube, add AgSbF in an argon atmosphere 6 (0.015mmol), amidine (1) (Ar 1 Is 4-chlorophenyl 0.2mmol), amidine (1’) (Ar 2 Is 4-bromophenyl 0.2mmol) and benzyl isonitrile (2) (0.3mmol), then add the solvent toluene (3mL), stir under heating at 140℃ for about 10 hours, stop the reaction, cool to room temperature, and remove the reaction solution Transfer to a rotary steaming flask, remove the solvent by distillation under reduced pressure, dissolve the residue in dichloromethane and load the sample, use 300-400 mesh silica gel as the stationary phase, and use petroleum ether: ethyl acetate = 50:1 (volume ratio) The mixed solution is used as the eluent for silica gel column chromatography, nuclear magnetic and liquid mass spectrometry detection, and 2,4-disubstituted-1,3,5 triazine (Ar 1 ...
Embodiment 2
[0033]
[0034] 2,4-Bis(4-methoxyphenyl)-1,3,5-triazine(3b)
[0035] Yield: 46.3mg (82%), white solid. 1 H NMR(400MHz, CDCl 3 )δ9.07(s,1H), 8.54(d,J=7.4Hz,4H), 6.99(d,J=7.4Hz,4H), 3.86(s,6H); 13 C NMR(100MHz, CDCl 3 )δ170.6, 166.4, 166.1, 163.5, 131.0, 130.8, 128.3, 114.3, 114.1, 55.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com