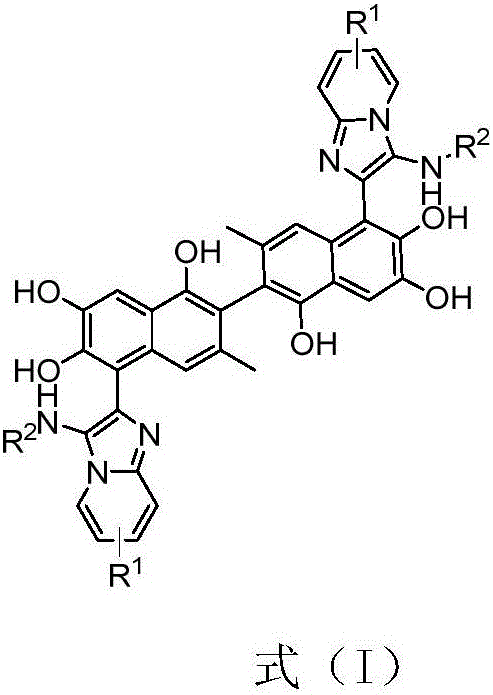
Apogossypol derivatives and preparation method thereof, and application of apogossypol derivatives in antitumor and immunoregulation
A technology of apogossypol and its derivatives, which is applied in the field of organic synthesis and medicine, and can solve the problems of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
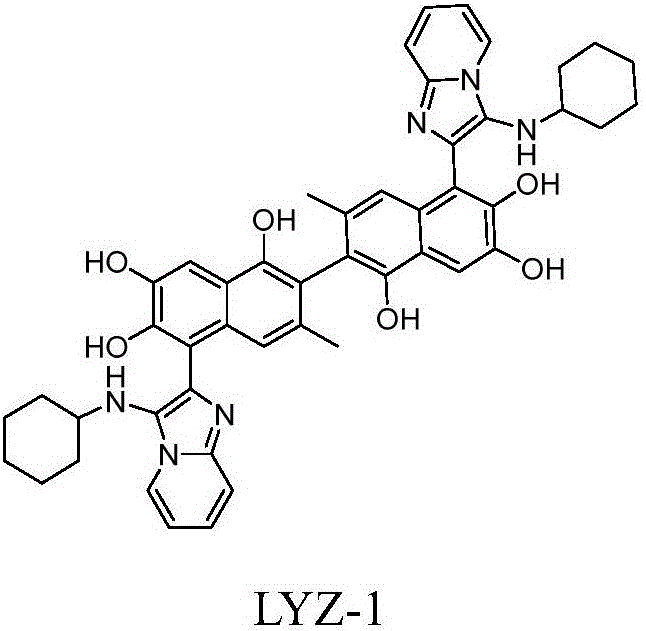
[0027] 5,5'-bis{3-(cyclohexylamine)imidazo[1,2-a]pyridyl}-3,3'-dimethyl-[2,2'-binaphthyl]-1,1 Preparation of ',6,6',7,7'-hexahydroxyl (LYZ-1)
[0028]
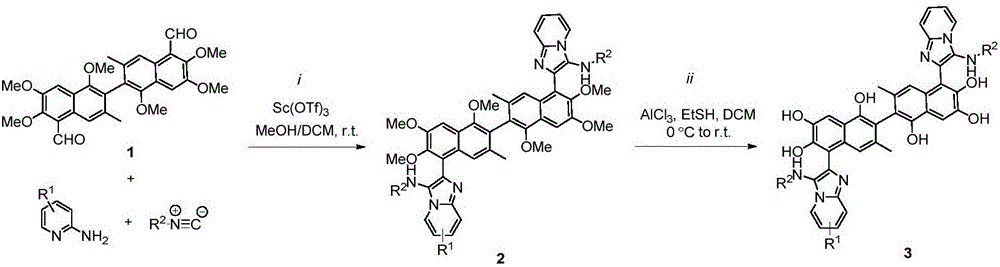
[0029] Weigh compound 1 (103mg, 0.2mmol, 1.0eq), 2-aminopyridine (45mg, 0.48mmol, 2.4eq), scandium trifluoromethanesulfonate (Sc(OTf) 3) (10mg, 0.02mmol, 0.1eq) in a round bottom flask was dissolved by adding 0.5mL of anhydrous DCM / MeOH (v / v=2:1) solution. After 30min, cyclohexaneisonitrile (52mg, 0.48mmol, 2.4eq) was added and stirred at room temperature. The reaction was monitored by TLC, and the product was separated by column chromatography as the raw material for the next reaction. Under anhydrous and oxygen-free conditions, ethanethiol 2mL was added to anhydrous aluminum chloride (667mg, 5.0mmol, 25.0eq). After cooling to 0°C, the product obtained in the previous step (180mg, 0.2mmol, 1.0eq) was dissolved in anhydrous dichloromethane and added dropwise to the above solution. After 3 hours, add water to quench th...
Embodiment 2
[0032] 5,5'-bis{3-(cyclohexylamine)-7-methylimidazo[1,2-a]pyridyl}-3,3'-dimethyl-[2,2'-binaphthyl Preparation of ]-1,1',6,6',7,7'-hexahydroxyl (LYZ-2)
[0033]
[0034] The preparation method is as in Example 1, from compound 1 (103mg, 0.2mmol, 1.0eq), 4-methyl-2-aminopyridine (52mg, 0.48mmol, 2.4eq), scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (10mg, 0.02mmol, 0.1eq), cyclohexane isonitrile (52mg, 0.48mmol, 2.4eq) prepared. Yellow-brown solid, yield 95%.
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ14.19(s,1H),14.11(s,1H),10.23(s,1H),10.18(s,1H),9.58(s,1H),9.52(s,1H),8.83–8.61(m ,2H),8.45–8.08(m,2H),7.71(d,J=4.0,2H),7.69(s,1H),7.66(s,1H),7.38(t,J=7.6,2H),6.83 (s,1H),6.82(s,1H),2.92–2.79(m,1H),2.68–2.61(m,1H),2.56(s,6H),1.83(s,3H),1.78(s,3H ),1.71–1.64(m,2H),1.59–1.47(m,4H),1.45–1.20(m,6H),1.09–0.91(m,6H),0.64–0.52(m,2H). 13 C NMR (100MHz, DMSO-d 6 )δ149.12,149.08,149.01,146.99,146.95,146.86,144.09,144.02,142.98,136.01,133.95,133.86,128.96,128.93,128.79,128.75,128.67,12...
Embodiment 3
[0037] 5,5'-bis{3-(cyclohexylamine)-7-ethylimidazo[1,2-a]pyridyl}-3,3'-dimethyl-[2,2'-binaphthyl Preparation of ]-1,1',6,6',7,7'-hexahydroxyl (LYZ-3)
[0038]
[0039] The preparation method is as in Example 1, from compound 1 (103mg, 0.2mmol, 1.0eq), 4-ethyl-2-aminopyridine (59mg, 0.48mmol, 2.4eq), scandium trifluoromethanesulfonate (Sc(OTf) 3 ) (10mg, 0.02mmol, 0.1eq), cyclohexane isonitrile (52mg, 0.48mmol, 2.4eq) prepared. Reddish-brown solid, yield 96%. 1 H NMR (400MHz, DMSO-d 6 )δ14.26(s,1H),14.16(s,1H),10.26(s,1H),10.22(s,1H),9.60(s,1H),9.54(s,1H),8.76(s,1H ),8.75(s,1H),8.35–8.24(m,1H),8.19–8.05(m,1H),7.72(t,J=4.3,4H),7.69(s,1H),7.65(s,1H ),7.45(t,J=6.6,2H),6.84(d,J=4.6,2H),2.87(dd,J=14.6,7.2,4H),2.74–2.57(m,2H),1.84(s, 3H),1.80(s,3H),1.75–1.62(m,4H),1.58–1.50(m,4H),1.42–1.36(m,2H),1.30(t,J=7.4,6H),1.06– 0.90(m,7H),0.69–0.49(m,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com