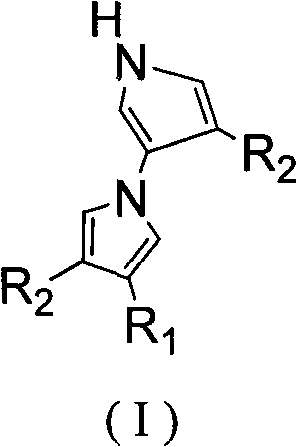
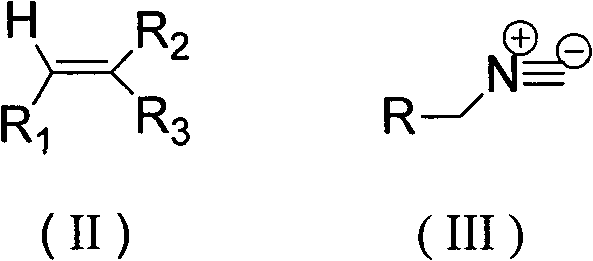
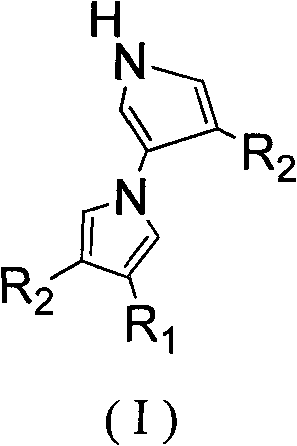
Method for tandem synthesis of dipyrrole and its derivatives through one-pot process
A technology for derivatives, dipyrrole, applied in the field of preparing dipyrrole and its derivatives, can solve the problems of difficult reaction conditions, high cost, long reaction route, etc. of raw materials, and achieves mild reaction conditions, good antibacterial activity, and good antitumor active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Under nitrogen protection, the raw material ethyl-2-cyano-3-ethoxypropene ester: TosMIC: NaH in molar ratio (1:1.2:1.3) was mixed and added to 10 mL of anhydrous CH 3 In CN, the reaction was stirred at room temperature for 12h, and the reaction was completed, and the reaction solution was washed with saturated NH 4 Cl solution to adjust the pH value to 7-8, then extract with dichloromethane 10mL×3, combine the organic layers, and wash with 10% Na 2 CO 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography to obtain the product 4-ethoxy-1'H-[1,3'-dipyrrole]-3,4'- Dinitrile, the product is a white solid, the yield is 53%.
Embodiment 2
[0026] Under nitrogen protection, the raw material ethyl-2-cyano-3-ethoxypropenyl ester:TosMIC:Cs 2 CO 3 According to the molar ratio (1:1.2:1.3) mixed into 10mL of anhydrous CH 3 In CN, the reaction was stirred at room temperature for 12h, and the reaction was completed, and the reaction solution was washed with saturated NH 4 Cl solution to adjust the pH value to 7-8, then extract with dichloromethane 10mL×3, combine the organic layers, and wash with 10% Na 2 CO 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography to obtain the product 4-ethoxy-1'H-[1,3'-dipyrrole]-3,4'- Dinitrile, the product is a white solid, the yield is 49%.
Embodiment 3
[0028] Under nitrogen protection, the raw material ethyl-2-cyano-3-ethoxypropenyl ester: TosMIC: K 2 CO 3 According to the molar ratio (1:1.2:1.3) mixed into 10mL of anhydrous CH 3 In CN, the reaction was stirred at room temperature for 12h, and the reaction was completed, and the reaction solution was washed with saturated NH 4 Cl solution to adjust the pH value to 7-8, then extract with dichloromethane 10mL×3, combine the organic layers, and wash with 10% Na 2 CO 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography to obtain the product 4-ethoxy-1'H-[1,3'-dipyrrole]-3,4'- Dinitrile, the product is a white solid, the yield is 28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com