Optically-active helix chain poly(phenyl isocyanide) and polymerization method thereof
An optically active, polyphenylene isonitrile technology, applied in the field of optically active helical chain polyphenylene isonitrile and its polymerization, can solve problems such as complex chiral structures, and achieve the effects of simplified synthesis steps, simple synthesis and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
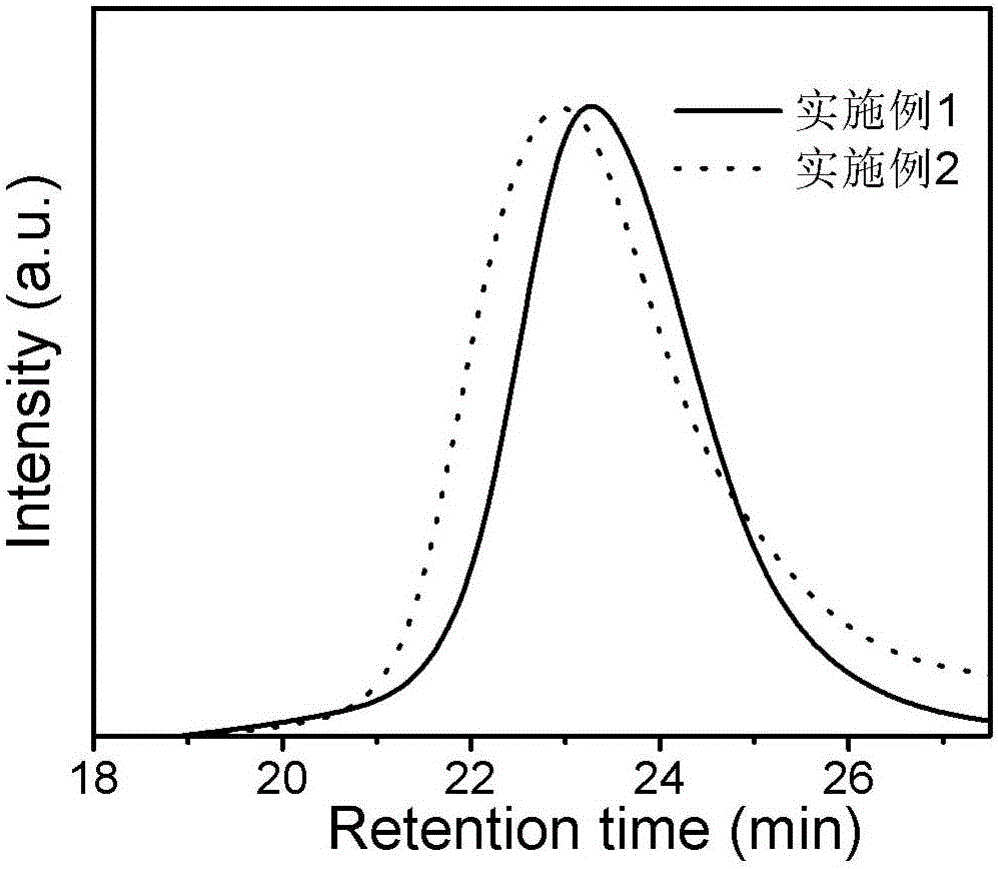
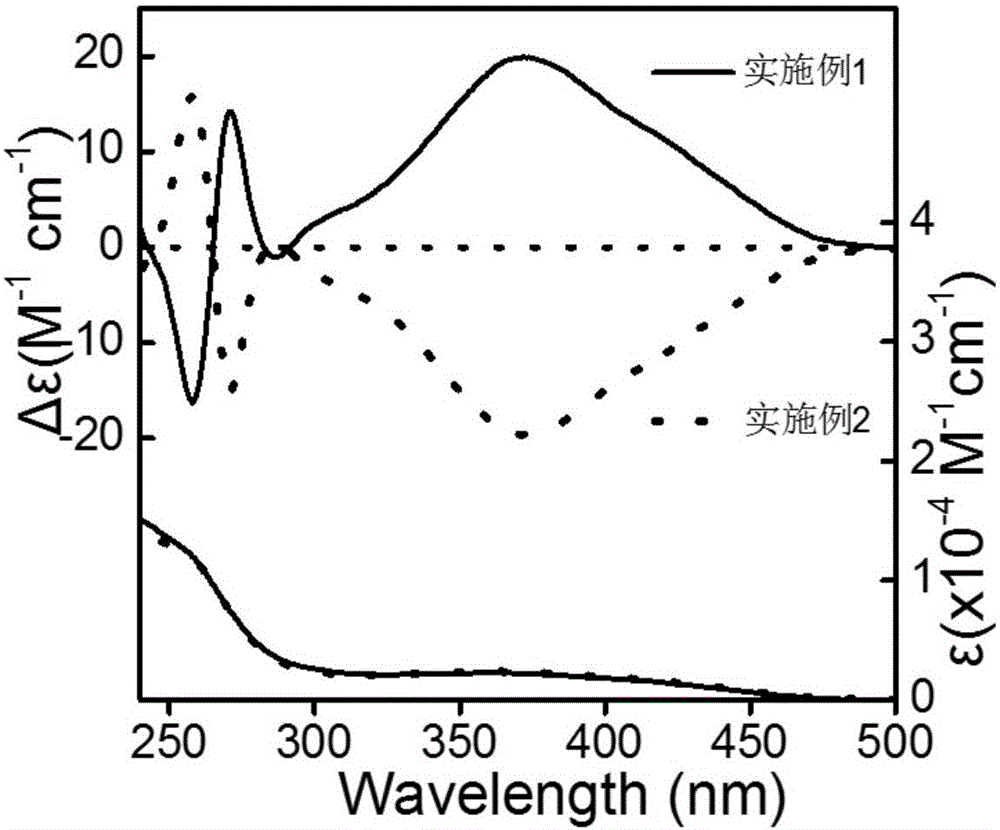
Embodiment 1
[0032] A kind of polymerization method of optically active helical chain polyphenylisonitrile, operates according to the following steps:
[0033] 1. Chiral phosphine ligand (S-BINAP) and palladium catalyst are carried out under strict anhydrous and oxygen-free conditions, and chiral phosphine ligand (S-BINAP) and palladium catalyst are added in a 10mL polymerization bottle, S-BINAP and The molar ratio of the palladium catalyst was 12:1, vacuumed and filled with nitrogen three times, added 0.3 mL of dry tetrahydrofuran, and stirred at room temperature for 3 h to obtain a mixture of S-BINAP and palladium catalyst.
[0034] 2. Weigh 0.1mmol (34mg) achiral benzene isonitrile monomer into a 10mL polymerization bottle, then add 0.8mL dry CHCl 3 Dissolved, then weighed 0.9 μmol of the mixed solution of S-BINAP and palladium catalyst, put it into the polymerization bottle under nitrogen atmosphere, reflux reaction at 55 ° C for 20 h, added 10 mL of methanol to quench, and precipitate...
Embodiment 2
[0036] A kind of polymerization method of optically active helical chain polyphenylisonitrile, operates according to the following steps:
[0037] 1. Chiral phosphine ligand (R-BINAP) and palladium catalyst are carried out under strict anhydrous and oxygen-free conditions, add chiral phosphine ligand (R-BINAP) and palladium catalyst in 10mL polymerization bottle, R-BINAP and The molar ratio of the palladium catalyst was 12:1. Vacuumize and inflate with nitrogen three times, add 0.3 mL of dry tetrahydrofuran, and stir at room temperature for 3 h to obtain a mixture of R-BINAP and palladium catalyst.
[0038] 2. Weigh 0.1mmol (34mg) achiral benzene isonitrile monomer into a 10mL polymerization bottle, then add 0.8mL dry CHCl 3 Dissolved, then weighed 0.9 μmol of the mixed solution of R-BINAP and palladium catalyst, put it into the polymerization bottle under nitrogen atmosphere, refluxed at 55 ° C for 20 h, added 10 mL of methanol to quench, and precipitated the polymer, washed ...
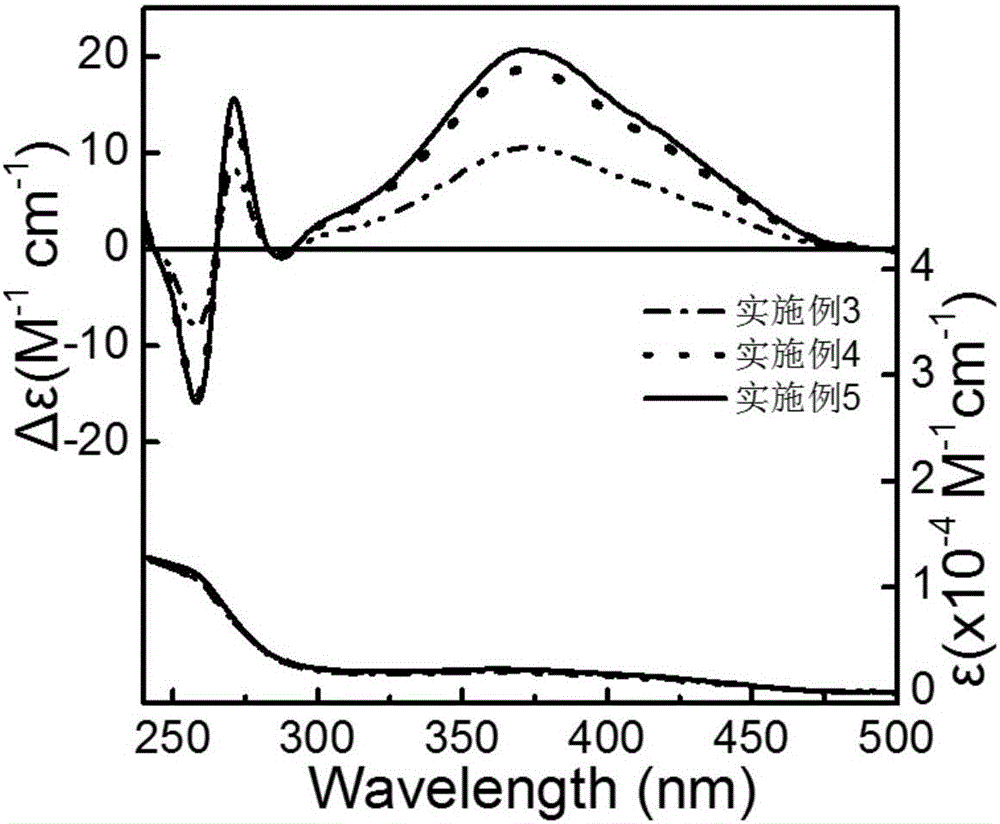
Embodiment 3
[0040] A kind of polymerization method of optically active helical chain polyphenylisonitrile, operates according to the following steps:
[0041] 1. Chiral phosphine ligand (S-BINAP) and palladium catalyst are carried out under strict anhydrous and oxygen-free conditions, and chiral phosphine ligand (S-BINAP) and palladium catalyst are added in a 10mL polymerization bottle, S-BINAP and The molar ratio of the palladium catalyst was 3:1. Vacuumize and inflate with nitrogen four times, add 0.3 mL of dry tetrahydrofuran, and stir at room temperature for 3 h to obtain a mixture of the palladium catalyst and S-BINAP.
[0042] 2. Weigh 0.1mmol (34mg) achiral benzene isonitrile monomer into a 10mL polymerization bottle, then add 0.8mL dry CHCl 3Dissolved, then weighed 0.9 μmol of the mixed solution of S-BINAP and palladium catalyst, put it into the polymerization bottle under nitrogen atmosphere, reflux reaction at 55 ° C for 20 h, added 10 mL of methanol to quench, and precipitated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com