Polythiourea compound ands preparation method and application thereof
A compound and a technology for polythiourea, applied in the field of polythiourea compounds and their preparation, can solve the problems of limited types of isothiocyanates, expensive imidazole monomers, generation of by-products, etc. The effect of adaptability and high polymerization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
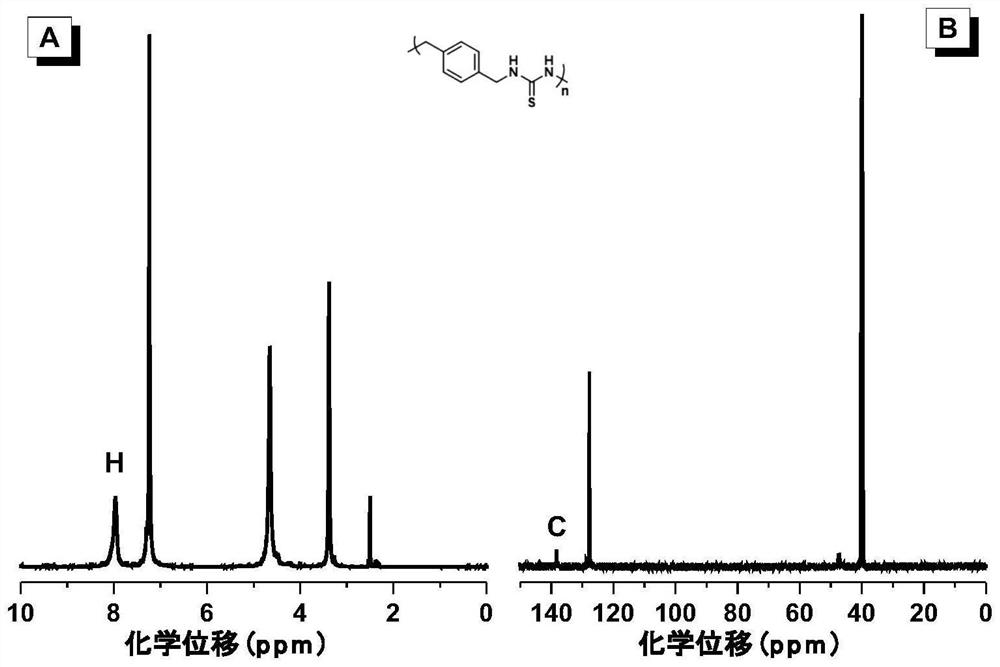
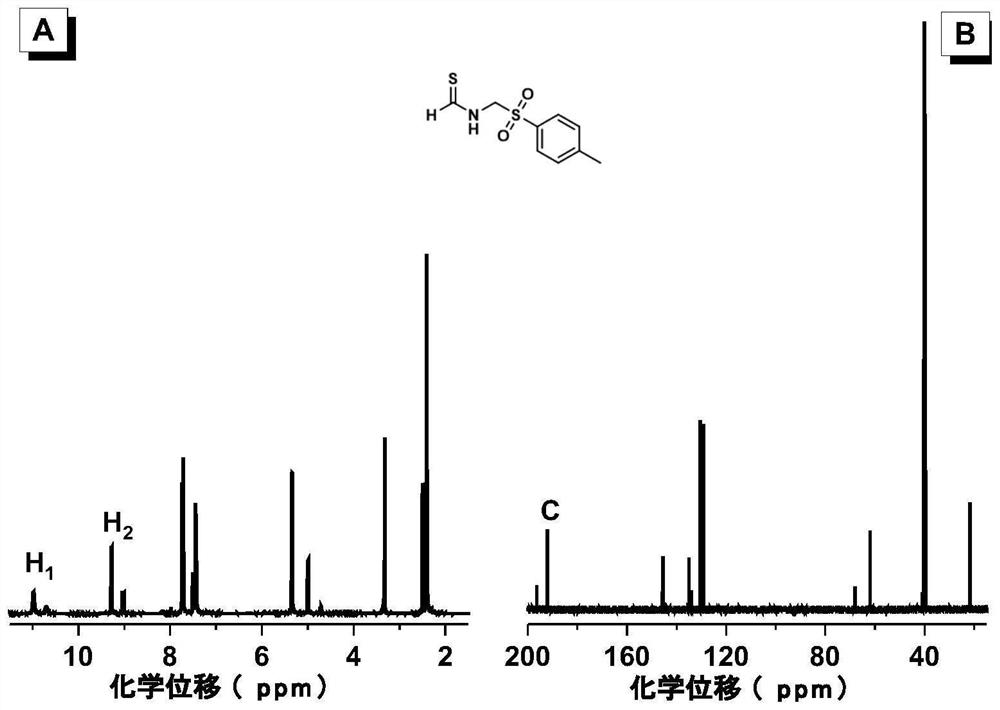
[0052] A symmetrical polythiourea compound whose structural formula is shown in P1; a thioformamide compound whose structural formula is shown in M1:
[0053]
[0054] The polythiourea compound and thioformamide compound are prepared by direct one-pot reaction of isonitrile, amine and carbon disulfide, and the reaction equation is as formula (2):
[0055]
[0056] Wherein, the monomers used can be purchased from the market. 1a is 1,4-xylylenediamine, purchased from Anaiji Chemical in this example. 2 is p-toluenesulfonylmethyl isonitrile, purchased from Biide Pharmaceutical Technology Co., Ltd. in this example. In this example, carbon disulfide was also purchased from Anaiji Chemical.
[0057] The preparation steps of described polythiourea compound and thioformamide compound are as follows:
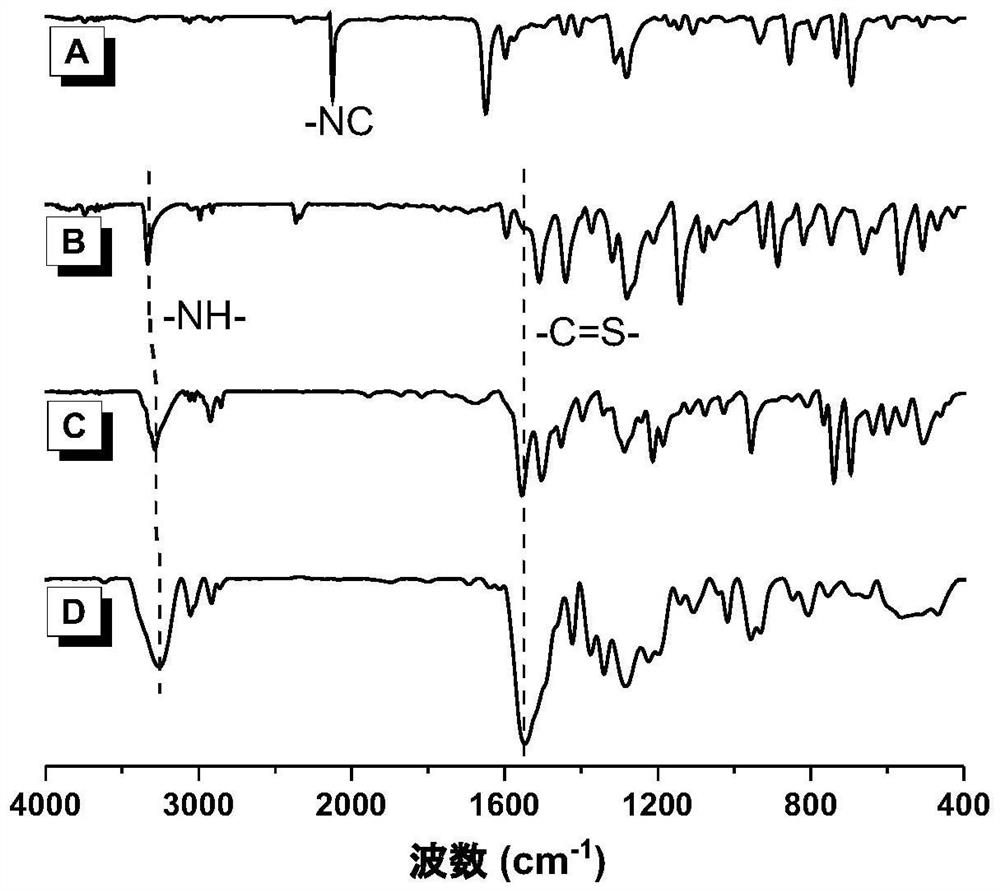
[0058] Add monomers 1a (136mg, 1mmol) and 2 (195mg, 1mmol) sequentially into a 10ml polymerization tube, then add 2mL dimethyl sulfoxide, stir at room temperature, after the mono...
Embodiment 2
[0061] A symmetrical polythiourea compound whose structural formula is shown in P2, a thioformamide compound whose structural formula is shown in M1:
[0062]
[0063] The polythiourea compound and the thioformamide compound are prepared by direct one-pot reaction of isonitrile, amine and carbon disulfide, and the reaction equation is as formula (3):
[0064]
[0065] Wherein, the monomers used can be purchased from the market, and the monomer 1b is 1,2-bis(2-aminoethoxy)ethane, purchased from TCI Company in this example. 2 is p-toluenesulfonylmethyl isonitrile, purchased from Biide Pharmaceutical Technology Co., Ltd. in this example. In this example, carbon disulfide was purchased from Anaiji Chemical.
[0066] The preparation steps of described polythiourea compound and thioformamide compound are as follows:
[0067] Add monomers 1b (148mg, 1mmol) and 2 (195mg, 1mmol) sequentially into a 10ml polymerization tube, then add 2mL dimethyl sulfoxide, stir at room temperat...
Embodiment 3
[0070] A sequence-controllable asymmetric polythiourea compound whose structural formula is shown in P3, and a thioformamide compound whose structural formula is shown in M1:
[0071]
[0072] The polythiourea compound and thioformamide compound are prepared by a one-pot reaction of isonitrile, amine and carbon disulfide, and the reaction equation is as formula (4):
[0073]
[0074] Wherein, the monomers used can be purchased from the market. Monomer 1a is 1,4-xylylenediamine, purchased from Anaiji Chemicals in this example. Monomer 1c is 4,4'-diaminodiphenyl ether, purchased from TCI in this example. 2 is p-toluenesulfonylmethyl isonitrile, purchased from Biide Pharmaceutical Technology Co., Ltd. in this example. In this example, carbon disulfide was purchased from Anaiji Chemical.
[0075] The preparation steps of described polythiourea compound and thioformamide compound are as follows:
[0076] Add monomers 1a (136mg, 1mmol) and 2 (390mg, 2mmol) sequentially int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com