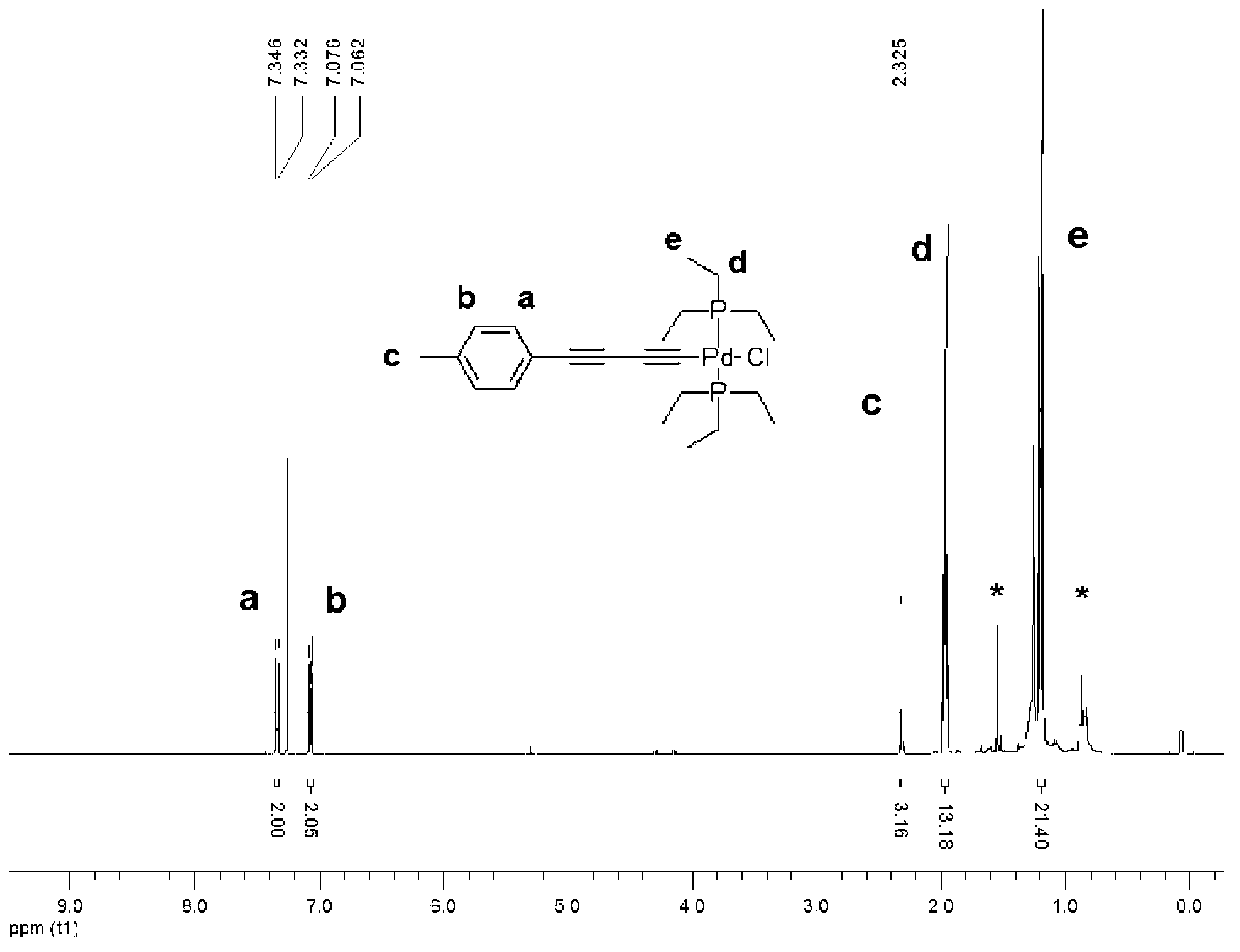Palladium catalyst, and preparation method and application thereof
A palladium catalyst and selected technology, applied in chemical instruments and methods, organic chemistry, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve the problem of poor catalytic controllability of divalent nickel salt, structural It is not easy to trim and modify, and the synthesis of rhodium complexes is difficult to achieve the effects of easy modification and trimming, narrow molecular weight distribution, and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of methyl substitution palladium catalyst
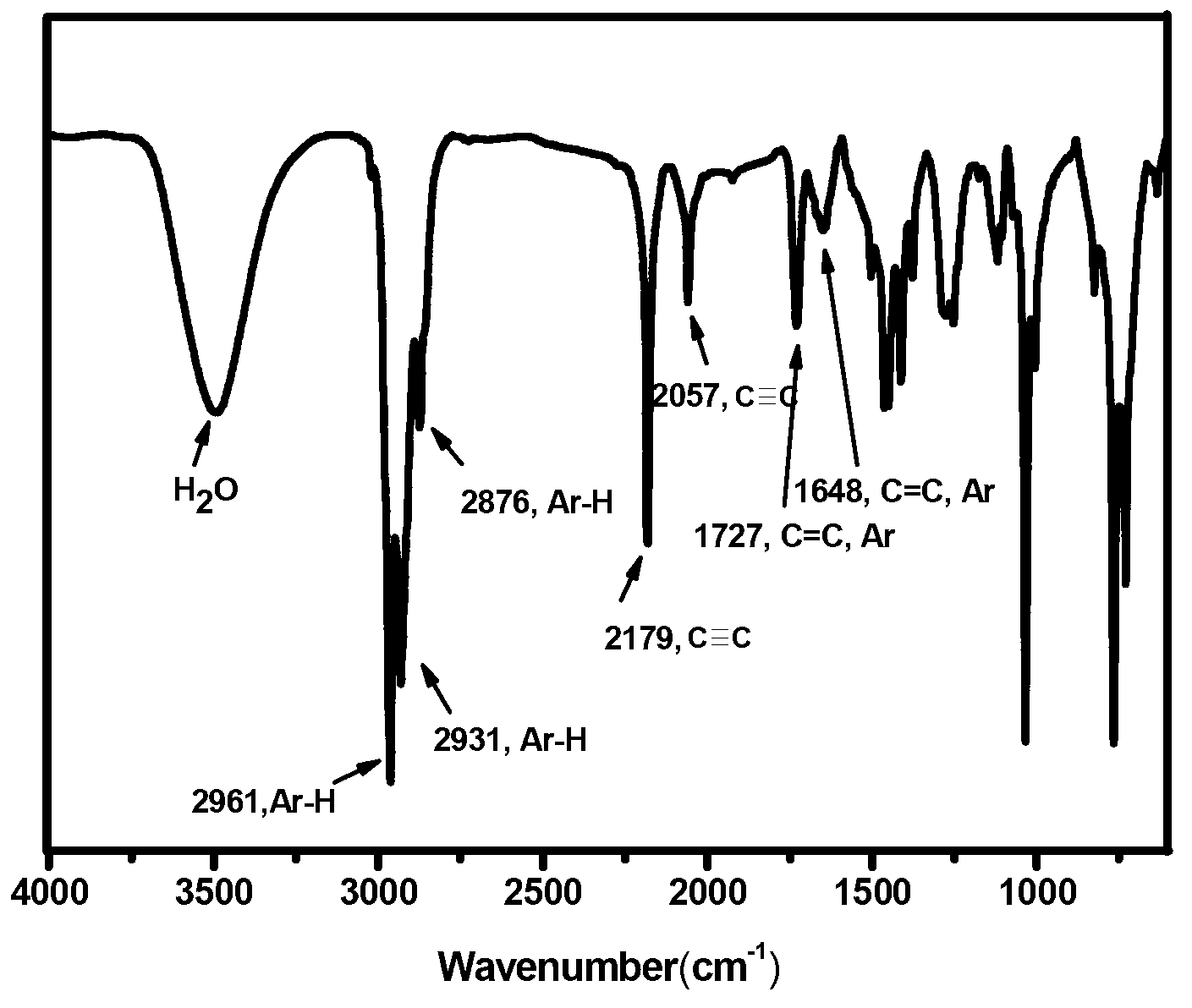
[0036]a. Using 1.73g (1.5mmol) of tetrakis(triphenylphosphine)palladium and 0.28g (1.5mmol) of copper iodide as catalysts, take 3.15g (32.1mmol) of trimethylsilylacetylene and 5.00g of 4-bromotoluene g (29.2mmol) was dissolved in dry 70mL triethylamine, reacted at 55°C in a nitrogen atmosphere for 20h, added 15mL water to quench the reaction, extracted the organic phase with 100mL ethyl acetate, followed by saturated ammonium chloride solution , saturated sodium bicarbonate solution, and saturated sodium chloride solution, washed 3 times, added anhydrous sodium sulfate to dry, and evaporated to remove the solvent to obtain a crude product. The crude product was subjected to silica gel column chromatography (eluent by volume ratio: petroleum ether: ethyl acetate ester = 4:1), purified and dried in vacuo to obtain a yellow oily liquid which is Intermediate I. The structural formula is as follows:
...
Embodiment 2
[0047] Embodiment 2: Initiate benzene isonitrile polymerization reaction
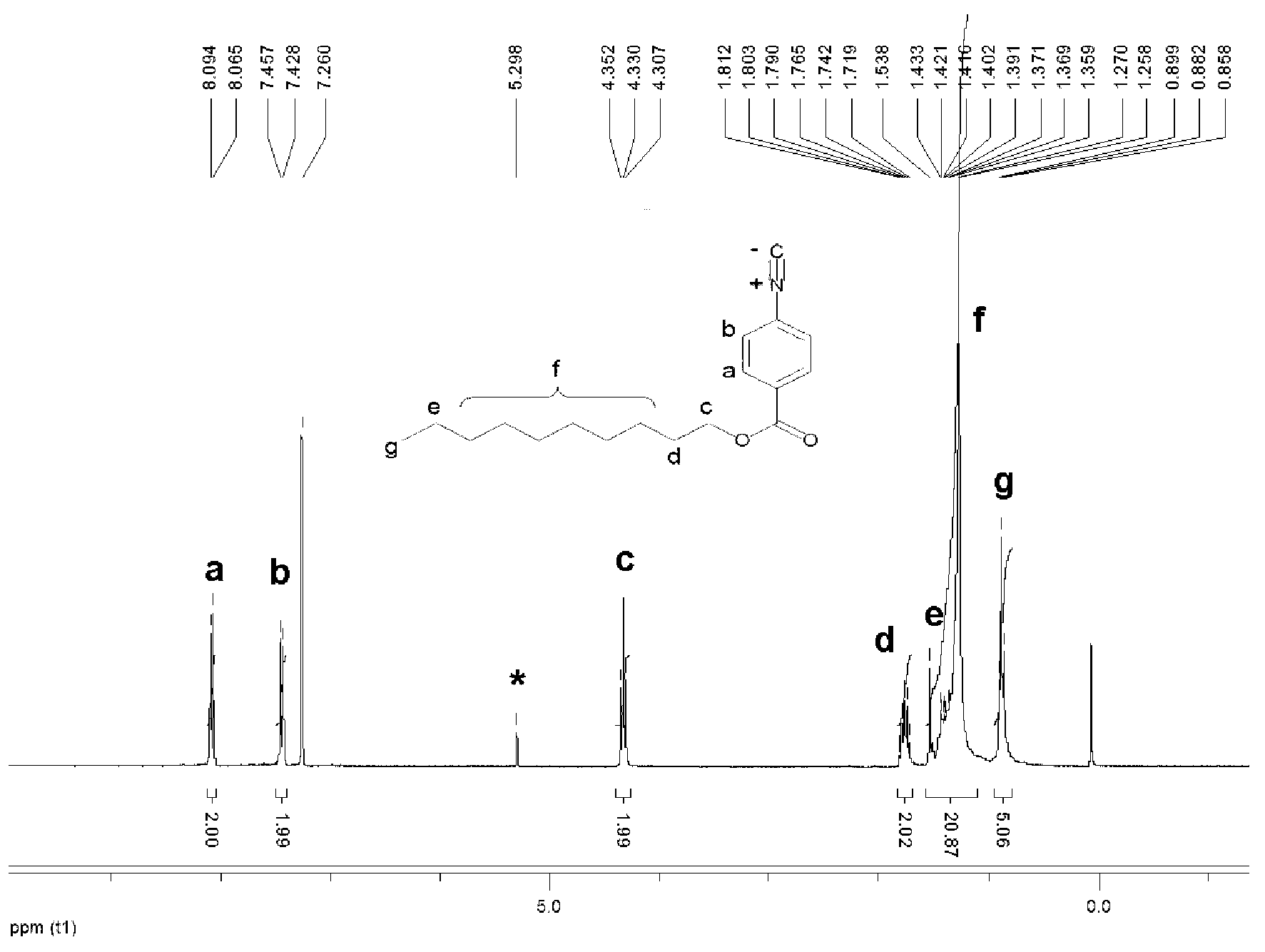
[0048] The polymerization of benzyl isonitrile is carried out under anhydrous and oxygen-free conditions. Add 2.5 μmol (1.3 mg) of the palladium catalyst prepared in Example 1 and 0.12 mmol (44.9 mg) of benzyl isonitrile monomer into a 10 mL polymerization bottle, vacuumize and fill with nitrogen to repeat 3 times, add 0.6mL of dry tetrahydrofuran, reflux at 55°C for 20h, add 10mL of methanol to quench, and precipitate the polymer, wash 5 times with methanol, centrifuge to obtain a yellow flocculent precipitate, and vacuum-dry until the mass is low. Change. Obtain 36.6mg polyisocyanide, its number-average molecular weight is 1.52×10 4 , the molecular weight distribution index is 1.19.
[0049] The structural formula of the benzene isonitrile monomer in the present embodiment is:
[0050]
Embodiment 3
[0051] Embodiment 3: Initiate benzene isonitrile polymerization reaction
[0052] The polymerization of benzene isonitrile is carried out under anhydrous and oxygen-free conditions. Add 2.1 μmol (1.1 mg) of palladium catalyst prepared in Example 1 and 0.15 mmol (41.6 mg) of benzene isonitrile monomer into a 10 mL polymerization bottle, vacuumize and fill with nitrogen to repeat 3 times, add 0.7 mL of dry tetrahydrofuran, reflux reaction at 55°C for 20 h, add 10 mL of methanol to quench, and precipitate the polymer, wash with methanol for 5 times, centrifuge to obtain yellow flocculent precipitate, and vacuum-dry until the mass is low. Change. Obtain 36.3mg polyisocyanide, its number average molecular weight is 2.06×10 4 , The molecular weight distribution index is 1.22.
[0053] The structural formula of the benzene isonitrile monomer in the present embodiment is:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com