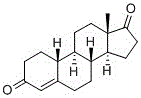
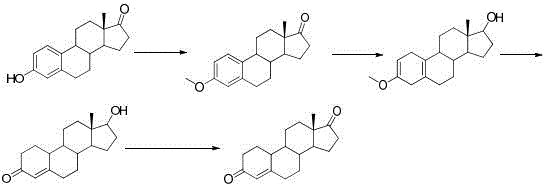
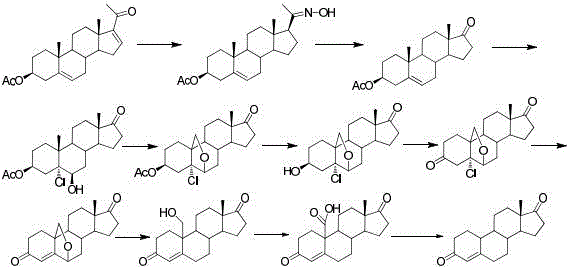
Method for preparing 19-nor-4-androstene-3,17-dione
A technology of androstene and diketone, which is applied in the field of chemical preparation, can solve the problems of restricting industrial production, long synthesis route, and long production cycle, and achieve the effects of improving operation safety, short synthesis route and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The first step, hydrolysis reaction: add compound 1 (150g, 1W) and tetrahydrofuran (750ml, 5V) into the three-necked flask, stir to dissolve, control the internal temperature at 20~25°C, prepare 4N HCl (187.5ml, 1.25V), transfer to drop Liquid funnel, drop into the three-necked flask, the temperature during the dropwise addition should not exceed 25°C, after the dropwise addition, keep the reaction at this temperature for 6 hours. Thin layer chromatography (TLC) detects that there is no raw material point, add 2N Na dropwise 2 CO 3 solution (187.5ml, 1.25V), adjust the pH to neutral, concentrate under reduced pressure at 40°C until solvent-free and drip, add ethyl acetate (300ml, 2V) to the aqueous layer to extract twice, combine the organic phases, and wash the organic phases with saturated salt Washed with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure at 50°C until solvent-free and dripped to obtain hydrolyzate 2 (110 g), with a mola...
Embodiment 2
[0045] The first step, hydrolysis reaction: Add compound 1 (150g, 1W) and ethanol (1500ml, 10V) to the three-necked flask, stir to dissolve, control the internal temperature at -10~-5°C, prepare 6N sulfuric acid (750ml, 5V), and transfer to drop Liquid funnel, drop into the three-necked bottle, the temperature during the dropwise addition should not exceed -10~-5°C, after the dropwise addition, keep the reaction at this temperature for 10 hours. When there is no raw material point detected by TLC, add 6N potassium carbonate solution (750ml, 5V) dropwise, adjust the pH to neutral, concentrate under reduced pressure at 40°C until there is no solvent, add ethyl acetate (1500ml, 10V) to the aqueous layer to extract two Once, the organic phases were combined, and the organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure at 50°C until solvent-free, and dropped to obtain hydrolyzate 2 (105 g), with a molar yield of 91...
Embodiment 3
[0049] The first step, hydrolysis reaction: Add compound 1 (150g, 1W) and methanol (150ml, 1V) to the three-neck flask, stir to dissolve, control the internal temperature at 55~60°C, prepare 0.5N HCl (150ml, 1V), and transfer to the drop solution Funnel, dropwise into the three-necked bottle, the temperature during the dropwise addition should not exceed 55~60°C, after the dropwise addition, keep the reaction at this temperature for 1 hour. When there is no raw material point detected by TLC, add 0.5N potassium hydroxide solution (150ml, 1V) dropwise, adjust the pH to neutral, concentrate under reduced pressure at 40°C until no solvent drops, add ethyl acetate (450ml, 3V) to extract the aqueous layer Twice, the organic phase was combined, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure at 50°C to solvent-free dropwise to obtain hydrolyzate 2 (112g), the molar yield was 97%, HPLC (High Performance Liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com