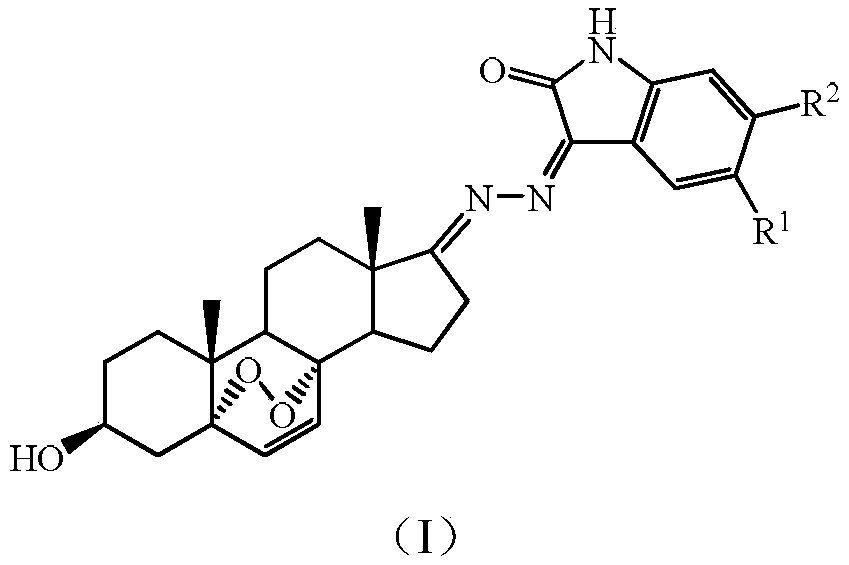
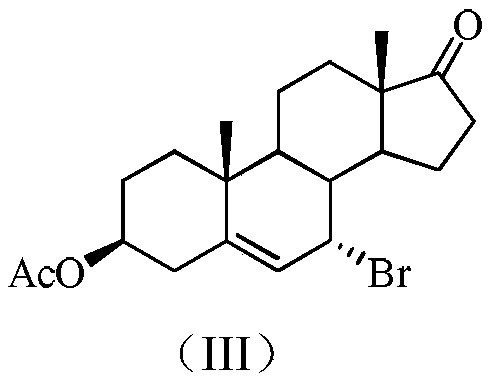
3beta-hydroxyl-5alpha,8alpha-peroxy-androstane-6-alkene-17-(isatin substituted) hydrazone derivative, as well as preparation and application thereof
A technology of peroxyandrosteroids and derivatives, which is applied in the application field of cancer and immune disease drugs, can solve the problems of drug resistance, toxic side effects, and restrictions on chemotherapeutic drugs, and achieve mild reaction conditions, proliferation inhibition, and simple steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 3β-hydroxy-5α,8α-peroxyandrost-6-ene-17-(isatin)hydrazone
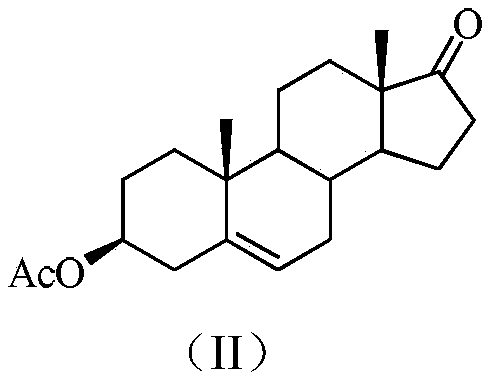
[0038] (a) Preparation of 3β-acetoxy-5-androsten-17-one
[0039]
[0040] The raw material dehydroepiandrosterone (28.8g, 0.1mol) was dissolved in a single-necked round bottom flask containing 100mL of dichloromethane and 20mL of pyridine, and 40mL of acetic anhydride was added dropwise with a constant pressure dropping funnel, and the dropping time was controlled at 20 minutes After dropping, the system was stirred and reacted for 4-6 hours, and the reaction process was detected by TLC. After the raw materials disappeared, the reaction was stopped. After adding 30mL of water and stirring, it was extracted with 40mL×3 ethyl acetate, and the organic phases were combined and washed with saturated sodium bicarbonate and Washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure to obtain 32 g of a white solid product with a yield of 98%....
Embodiment 2
[0067] Preparation of 3β-hydroxy-5α,8α-peroxyandrost-6-ene-17-(5-fluoro-isatin)hydrazone
[0068] Steps (a), (b), (c), (d), (e), (f) are the same as in Example 1.
[0069] (g) Preparation of 3β-hydroxy-5α,8α-peroxyandrost-6-ene-17-(5-fluoro-isatin)hydrazone
[0070]
[0071]3β-Hydroxy-5α,8α-peroxyandrost-6-ene-17-hydrazine (100mg, 0.30mmol) and 5-fluoro-isatin (54.5mg, 0.33mmol) obtained in step (f) were dissolved in Put 20mL of absolute ethanol in a 50mL single-necked flask, heat the above system to 50°C and react for 2-4 hours, wait until there is no raw material, and then end the reaction. Precipitation under reduced pressure and purification on a silica gel column gave 113.53 mg of a yellow solid product with a yield of 79%.
[0072] 1 H NMR (400MHz, CDCl 3 , δppm) 10.93(bs, 1H), 7.85(q, J=8Hz, 1H), 6.87-6.82(m, 1H), 6.70(q, J=8Hz, 1H), 6.55(d, J=8.4Hz, 1H), 6.33(d, J=8.4Hz, 1H), 4.62(d, J=8Hz, 1H), 2.48-1.95(m, 10H), 1.85-1.15(m, 10H), 1.01(s, 3H) , 0.99(s, 3H); ...
Embodiment 3
[0074] Preparation of 3β-hydroxy-5α, 8α-peroxyandrost-6-ene-17-(5-chloro-isatin)hydrazone
[0075] Steps (a), (b), (c), (d), (e), (f) are the same as in Example 1.
[0076] (g) Preparation of 3β-hydroxy-5α,8α-peroxyandrost-6-ene-17-(5-chloro-isatin)hydrazone
[0077]
[0078] 3β-Hydroxy-5α,8α-peroxyandrost-6-ene-17-hydrazine (100 mg, 0.30 mmol) and 5-chloro-isatin (59.7 mg, 0.33 mmol) obtained in step (f) were dissolved in Put 20mL of absolute ethanol in a 50mL single-necked flask, heat the above system to 50°C and react for 2-4 hours, wait until there is no raw material, and then end the reaction. Precipitation under reduced pressure and purification on a silica gel column yielded 120.3 mg of a yellow solid product with a yield of 81%.
[0079] 1 H NMR (CDCl 3 , 400MHz, δppm) 10.65(bs, 1H), 7.84(q, J=8Hz, 1H), 6.89-6.83(m, 1H), 6.72(q, J=8Hz, 1H), 6.55(d, J=8.4 Hz, 1H), 6.35(d, J=8.4Hz, 1H), 4.63(d, J=8Hz, 1H), 2.49-1.97(m, 10H), 1.84-1.15(m, 10H), 1.01(s, 3H), 0.99(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com