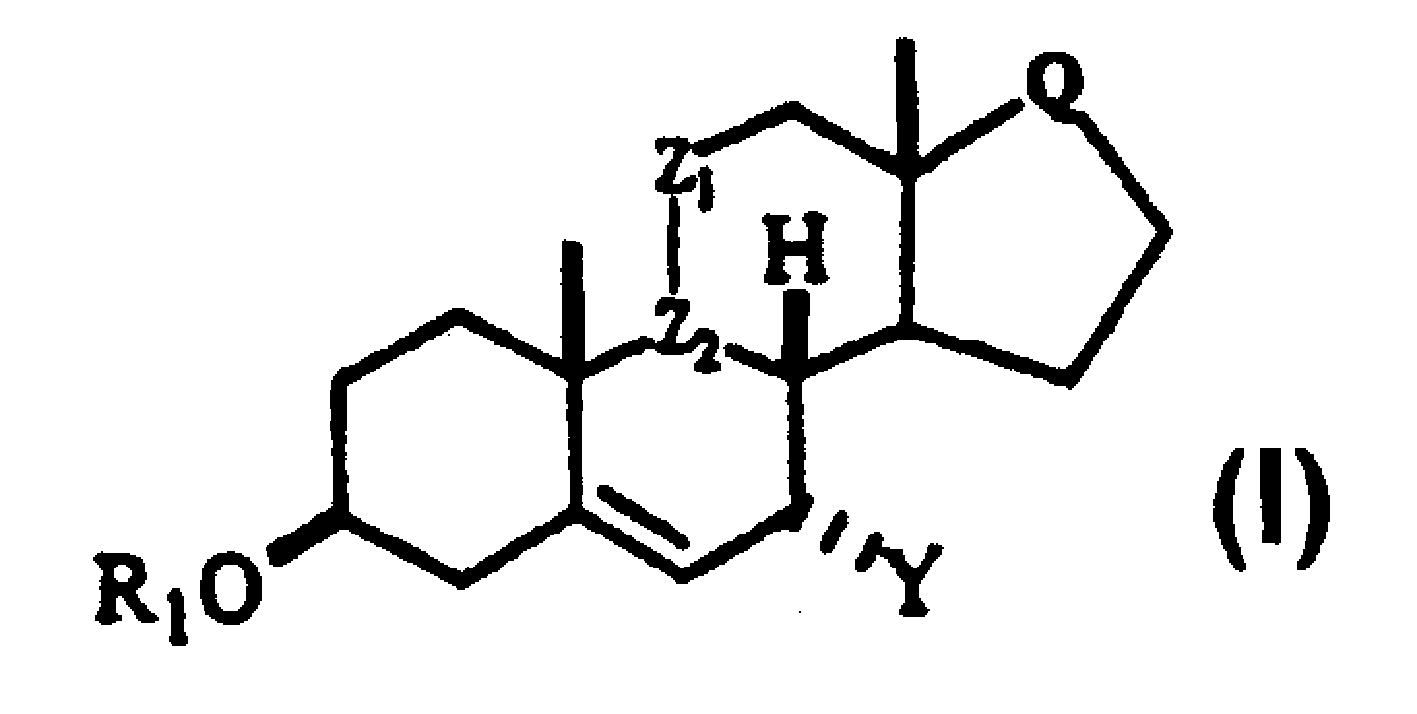
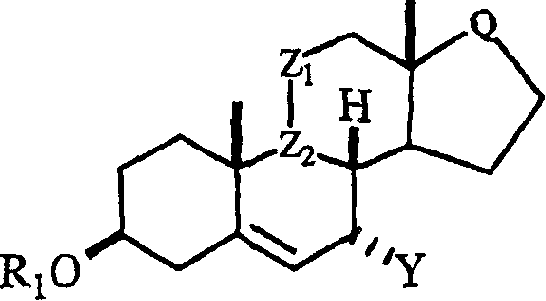
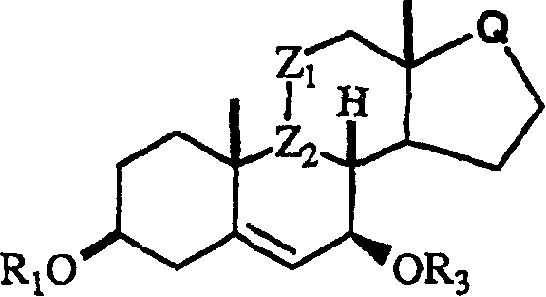
Processes for preparing C-7 substituted 5-androstenes
A steroid, CH2 technology, applied in androstane derivatives, chemical instruments and methods, steroids, etc., can solve problems such as difficulty in preparing steroids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: the preparation of tricarbonate 2
[0105] To 3N 250 ml RBF was added triol 1 (Scheme I) (10.00 g, 31 mmol) dissolved in pyridine (100 ml). To this solution was added triethylamine (31ml, 218mmol), methoxycarbonylbenzotriazole (24.2g, 125mmol) and 4-N,N-dimethylaminopyridine (1.2g, 9.4mmol). The resulting slurry was stirred for 2 hours at which time all had dissolved. Additional methoxycarbonylbenzotriazole (12g, 62mmol) and triethylamine (10ml, 73mmol) were added. Once the solids dissolve, the reaction is complete. Water (300ml) was added slowly and the mixture was cooled in an ice bath. The precipitate was filtered off, washed with 10% HCl (2 x 35 ml) and hexane (3 x 50 ml), and dried in a vacuum oven for 24 hours to afford the title compound 2 (Scheme I). 13 C NMR (CDCl 3 )δ 217.78, 155.60, 155.23, 154.88, 144.48, 122.35, 78.58, 76.81, 75.39, 55.29, 54.93, 51.09, 49.47, 47.79, 38.48, 37.89, 36.19, 36.08, 27.96, 29.3
Embodiment 2
[0106] Example 2: Preparation of furan 3 (Scheme 1)
[0107] A solution of tricarbonate 2 (1.0 g, 2.02 mmol) in 7 mL of acetonitrile was treated with 2-methylfuran (0.2 mL, 2.22 mmol) and 0.298 g Sc(OTf) at room temperature 3 Process for 1 hour. TLC (30% EtOAc / Hex) showed the reaction was complete. Chromatography on silica gel with 25% EtOAc / Hex afforded 0.92 g (96% yield) of furan 3 . 13 C NMR (CDCl 3 )δ 217.88,171.08,155.34,154.93,152.38,151.49,140.72,123.98,110.56,106.45,77.50,75.89,60.51,54.98,54.71,47.45,46.57,38.73,37.66,36.21,35.91,27.96,22.22,19.14, 13.98, 13.77.
Embodiment 3
[0108] Example 3: Preparation of Diol 4 (Scheme 1)
[0109] A solution of dicarbonate 3 (1.0 g) in 10 mL of MeOH was treated with 500 mg K2CO 3 For processing, heat to 40 °C. The mixture was stirred as TLC showed the reaction was complete. After the reaction was complete the slurry was poured into water, the product was isolated with EtOAc and the organics were concentrated to give diol 4 as a viscous oil.
[0110] 1 H NMR (CDCl 3 )δ 5.7(s, H), 5.45(d, J=5.7Hz, 1H), 3.45(m, 1H), 3.29(t, J=5.1Hz, 1H), 2.09(s, 3H), 1.1(s , 3H), 0.75(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com