Method for preparing 19- nor-4-androstene-3, 17-diketone
A technology of androstene and diketone, which is applied in the field of chemical synthesis of steroid hormone drugs, can solve the problems of long route, high cost, and large environmental pollution, and achieve the effects of high yield, short synthesis steps, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
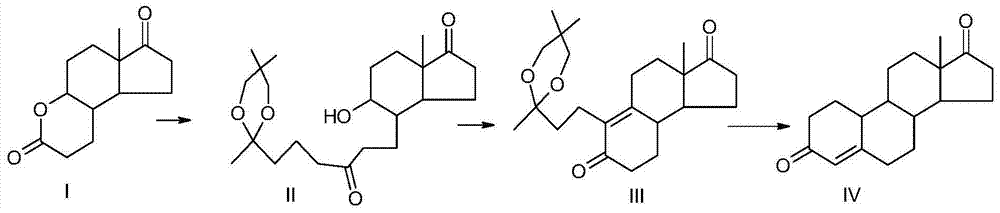
[0025] The preparation method of the present invention specifically comprises the following steps:
[0026] (1) Grignard reaction
[0027] (a) Under the protection of nitrogen, put magnesium into the first organic solvent at room temperature, add the ketal solution dropwise, and complete the dropwise addition in 4h-8h. After the dripping is completed, keep the temperature for 2h-4h, and then cool down naturally to 0°C for later use. Grignard reagent. In this step, the first organic solvent is anhydrous ether and / or tetrahydrofuran, preferably anhydrous ether; in the ketal solution, the ketal is 2-(3-chloropropyl)-2,5,5-tri Methyl-1,3-dioxane, 2-(3-bromopropyl)-2,5,5-trimethyl-1,3-dioxane or 2-(3- iodopropyl)-2,5,5-trimethyl-1,3-dioxane, more preferably 2-(3-chloropropyl)-2,5,5-trimethyl-1, 3-dioxane, the solvent is an ether solvent, preferably tetrahydrofuran; the reaction temperature during the heat preservation reaction is 20°C to 60°C, preferably 20°C to 40°C. The mass ...
Embodiment 1
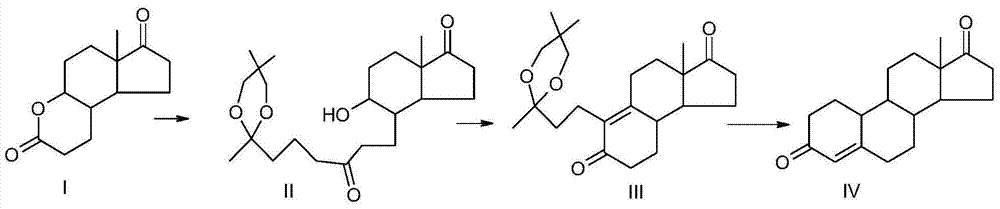
[0036] A preparation method of 19-nor-4-androstene-3,17-dione of the present invention, the preparation method uses compound I as a raw material, undergoes Grignard reaction, oxidation and ring closure reaction, reduction and ring closure reaction Prepared, the reaction scheme is as follows:
[0037]
[0038] (1) Grignard reaction
[0039] (a) At room temperature, under the protection of nitrogen, add 70ml of anhydrous ether and 8.5g of magnesium to a clean and dry 500ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and mechanical stirring, and heat up to 30°C. After the reaction starts, Start to drop the previously prepared 60g of ketal (specifically 2-(3-chloropropyl)-2,5,5-trimethyl-1,3-dioxane) and 50ml of tetrahydrofuran mixture , 5 to 6 hours after dripping, then keep warm for 3 hours, stir naturally for 4 hours, cool down to 0 degrees for later use, and obtain the Grignard reagent.
[0040] (b) Under nitrogen protection, add 175m...
Embodiment 2
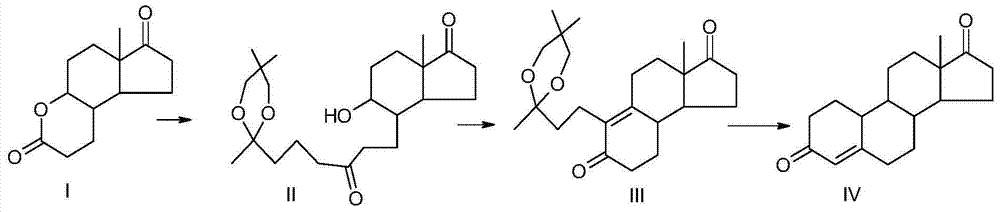
[0048] A preparation method of 19-nor-4-androstene-3,17-dione of the present invention, the preparation method uses compound I as a raw material, undergoes Grignard reaction, oxidation and ring closure reaction, reduction and ring closure reaction Prepared, the reaction scheme is as follows:
[0049]
[0050] The preparation method comprises the following steps:
[0051] (1) Grignard reaction
[0052] (a) At room temperature, under the protection of nitrogen, add 130ml of anhydrous ether and 17g of magnesium to a clean and dry 100ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and mechanical stirring, and heat up to 30°C. After the reaction starts, start 20 g of the ketal compound (specifically 2-(3-bromopropyl)-2,5,5-trimethyl-1,3-dioxane) prepared before was added dropwise and kept for 2 hours. Add 120g of ketal and 100ml of tetrahydrofuran dropwise, finish dropping in 5-6 hours, keep warm for 3 hours, stir naturally for 4 hours, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com