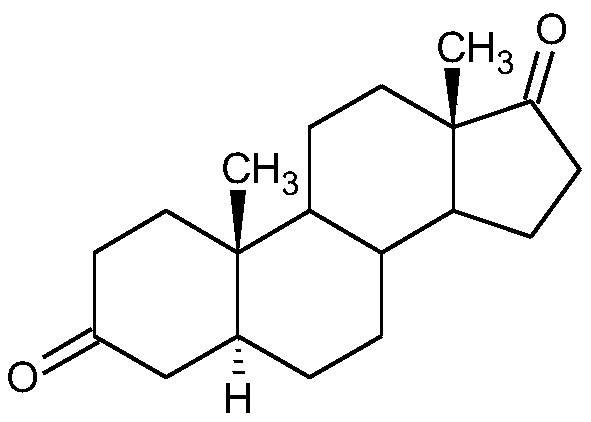
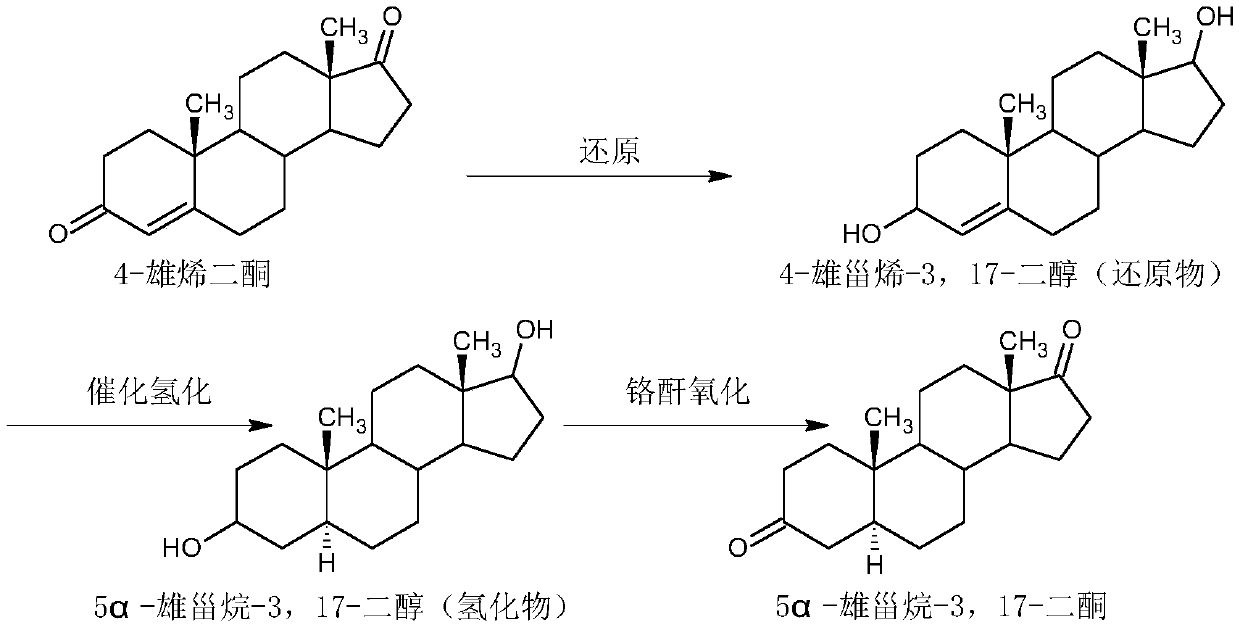
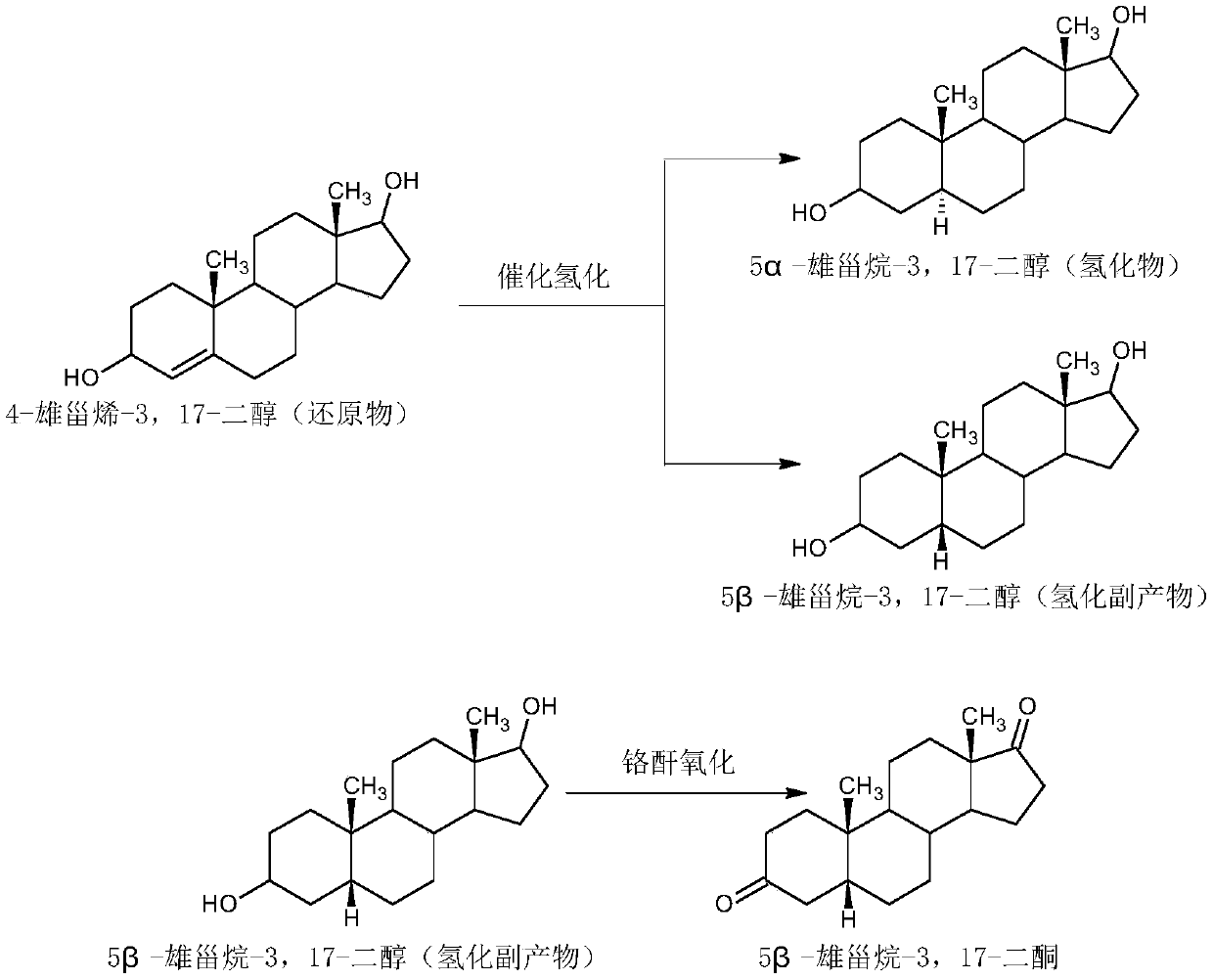
Preparation method of 5alpha-androstane-3,17-dione
A technology of androstane and androstane, which is applied in the field of preparation of 5α-androstane-3,17-dione, can solve the problems of high production cost, environmental pollution, by-product generation, etc., and achieve low production cost , The process route is short and the effect of ensuring the yield level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of 5α-androstane-3,17-dione, specifically comprising the following steps:
[0039] Step 1: Preparation of compound 3-ethoxy-androst-3,5-dien-17-one wet material: add 100g of 4-androstenedione, 60ml of triethyl orthoformate, 100ml of absolute ethanol into the reaction flask After the addition, stir for 30 minutes, then add 2.0 g of pyridine hydrobromide, heat up at 35°C to 45°C and keep warm for 6 hours for etherification reaction; after the reaction is completed, cool down to below 5°C, and drop triethylamine to adjust the pH 7.0 to 7.5, heat up and reflux for 1 hour after adding; cool down to below 0°C, filter to obtain 107.8g of compound 3-ethoxy-androst-3,5-dien-17-one wet material;
[0040] Step 2: Prepare a solution of 3-ethoxy-3-androstene-17-one: add 25g of 3-ethoxy-androstene-17-one obtained in the previous step to the reaction bottle -ketone wet material, 625ml methyl alcohol, 125ml dichloromethane, after stirring evenly, dropwise add the ...
Embodiment 2
[0043] A preparation method of 5α-androstane-3,17-dione, specifically comprising the following steps:
[0044] Step 1: Preparation of compound 3-ethoxy-androst-3,5-dien-17-one Wet material: add 100g of 4-androstenedione, 50ml of triethyl orthoformate, 120ml of absolute ethanol into the reaction flask After the addition, stir for 30 minutes, then add 1.5g of pyridine hydrobromide, heat up at 40°C to 50°C and keep warm for 7 hours for etherification reaction; after the reaction is completed, cool down to below 5°C, and drop triethylamine to adjust the pH value to 7.0 to 7.5, heat up and reflux for 1 hour after adding; cool down to below 0°C, filter to obtain 108.1g of compound 3-ethoxy-androst-3,5-dien-17-one wet material;
[0045] Step 2: Add 30g of 3-ethoxy-androst-3,5-dien-17-one wet material, 600ml of methanol, and 150ml of dichloromethane into the reaction bottle, stir well and add hydrogen dropwise The methanol solution of sodium oxide adjusts the pH value to 8.5-9.0, add...
Embodiment 3
[0048] A preparation method of 5α-androstane-3,17-dione, specifically comprising the following steps:
[0049] Step 1: Add 100g of 4-androstenedione, 100ml of triethyl orthoformate, and 90ml of absolute ethanol into the reaction flask, stir for 30 minutes after the addition, then add 2g of pyridine hydrochloride, heat up at 30°C to 40°C for 9 hours Carry out etherification reaction; after the reaction is completed, cool down to below 5°C, add dropwise triethylamine to adjust the pH value to 7.0-7.5, and then raise the temperature and reflux for 1 hour; cool down to below 0°C, and filter to obtain 106.9g of compound 3-ethoxy Base-androst-3,5-dien-17-one wet material.
[0050] Step 2: Add 25g of 3-ethoxy-androst-3,5-dien-17-one, 625ml of methanol, and 125ml of methylene chloride obtained in the previous step into the reaction bottle, stir well and add potassium hydroxide dropwise Adjust the pH value of the methanol solution to 7.5-8.0, add 2.5g of palladium-carbon catalyst with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com