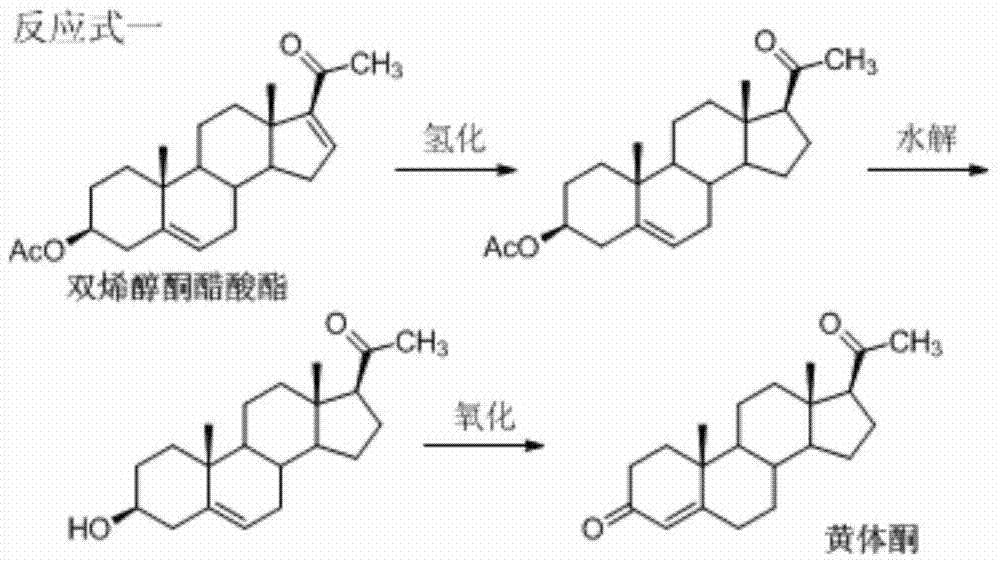
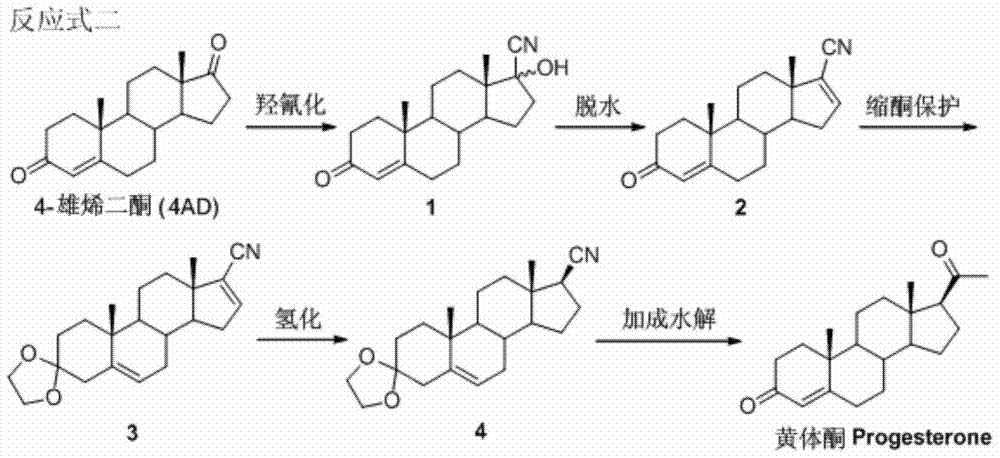
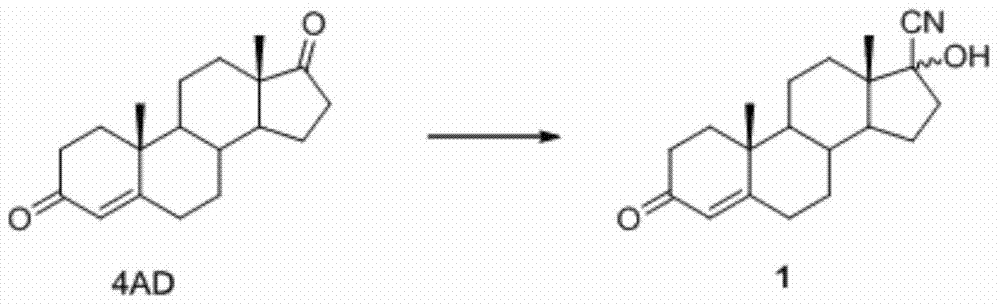
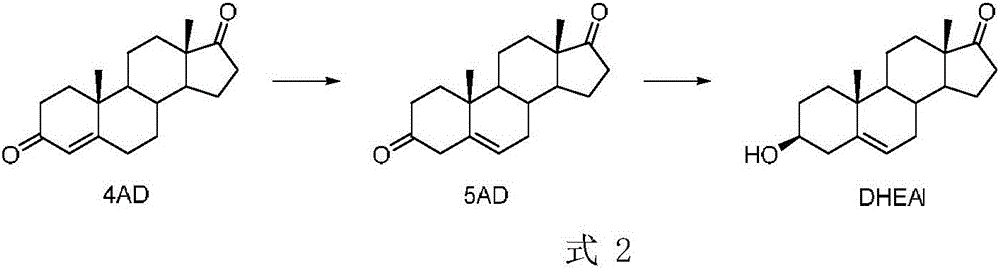
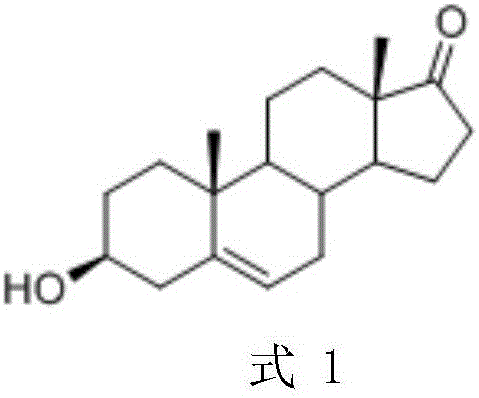
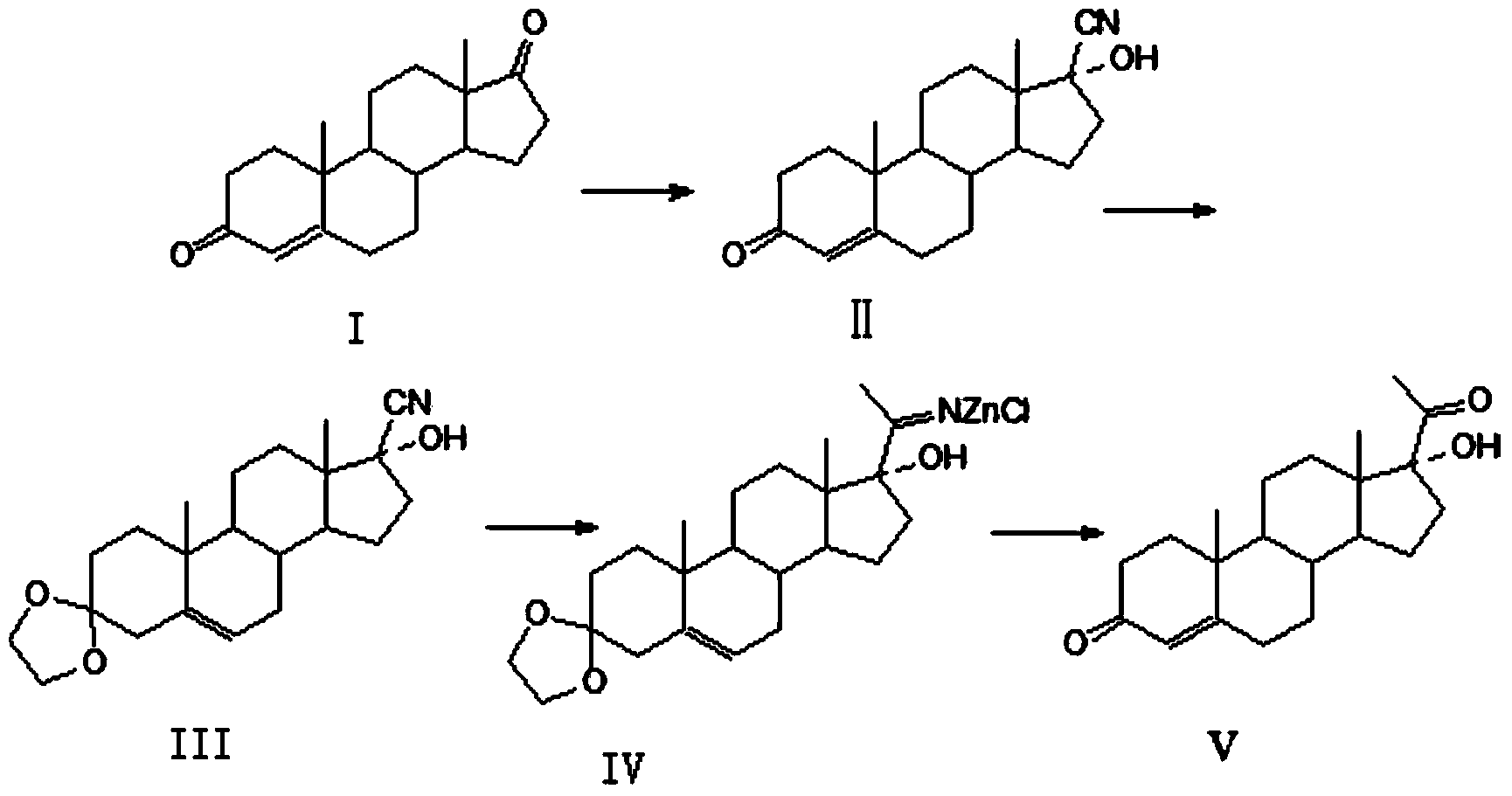
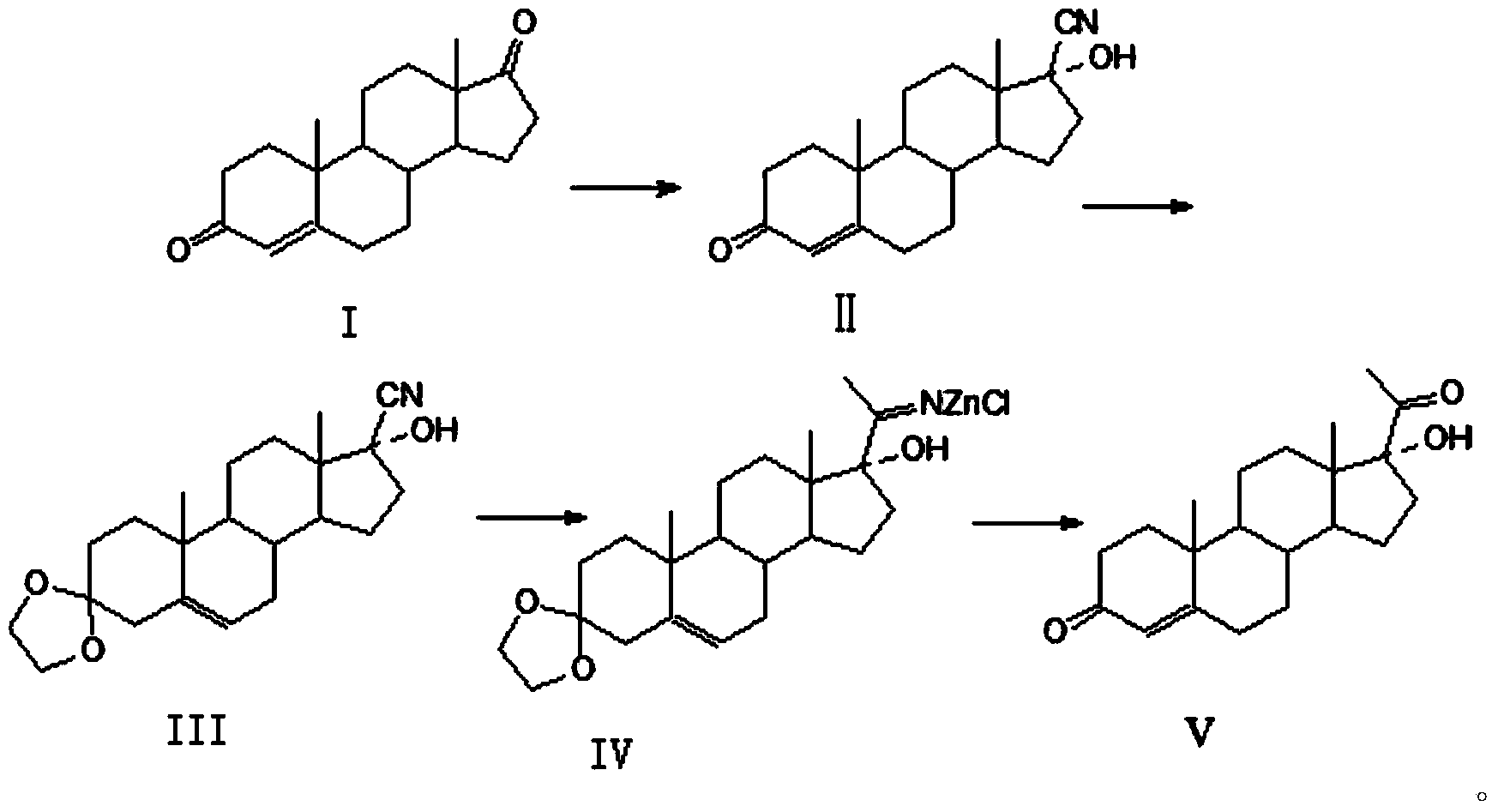
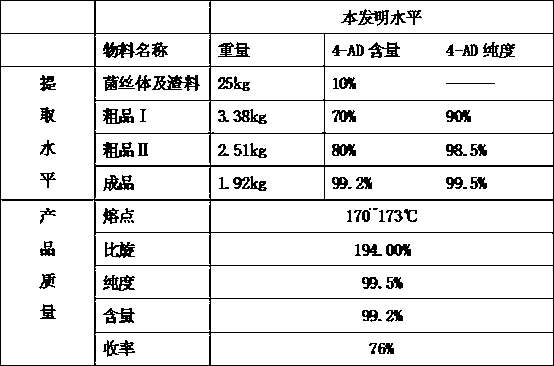
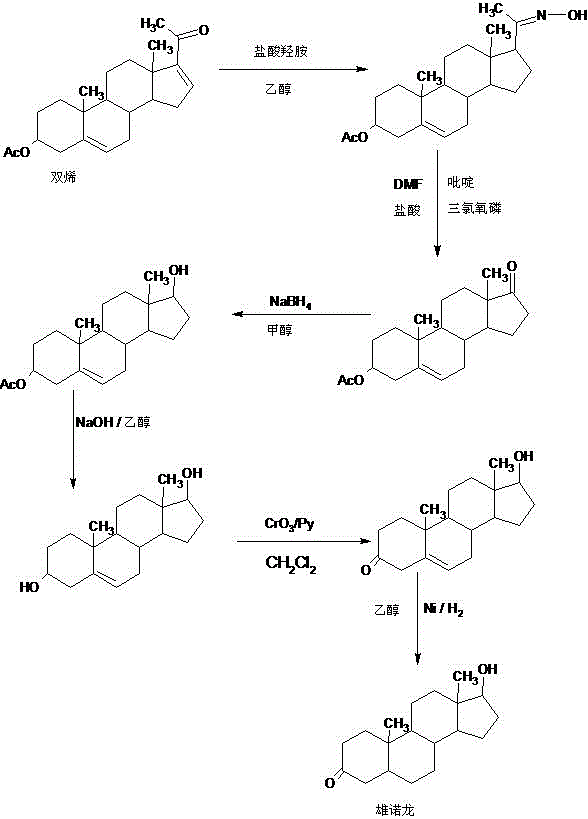
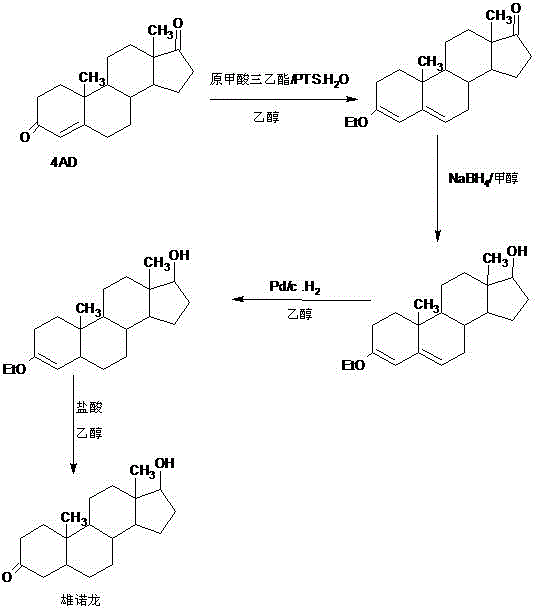
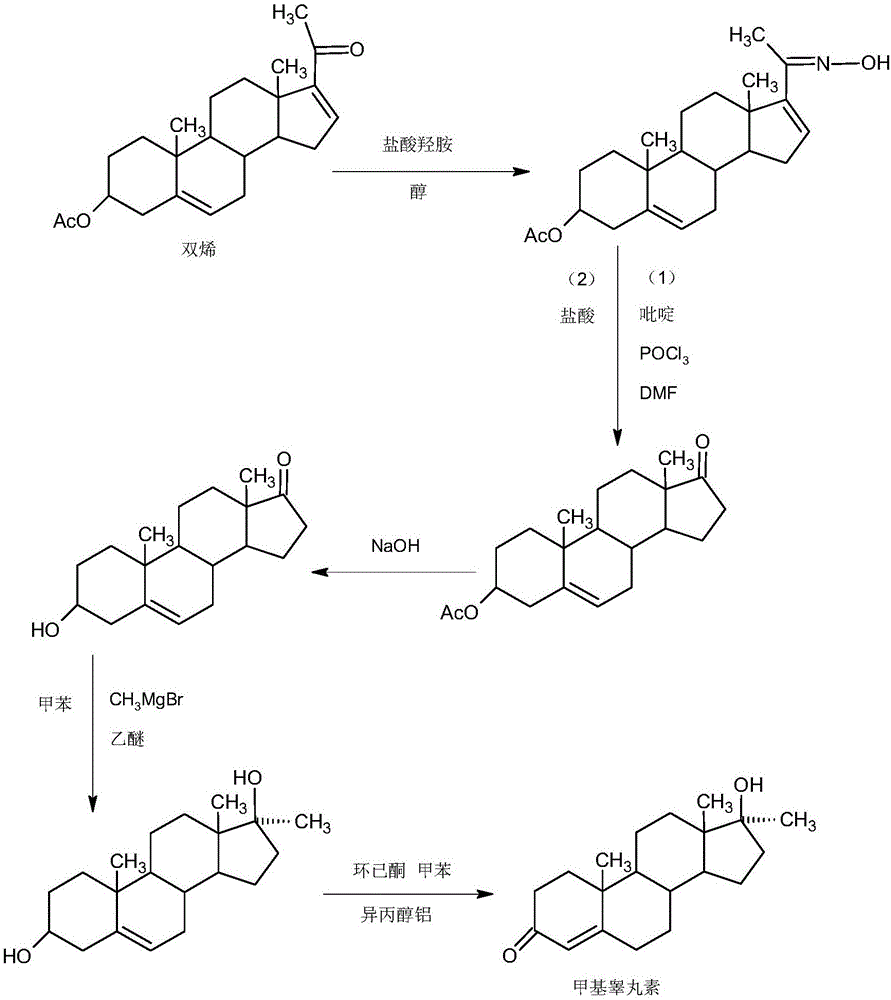
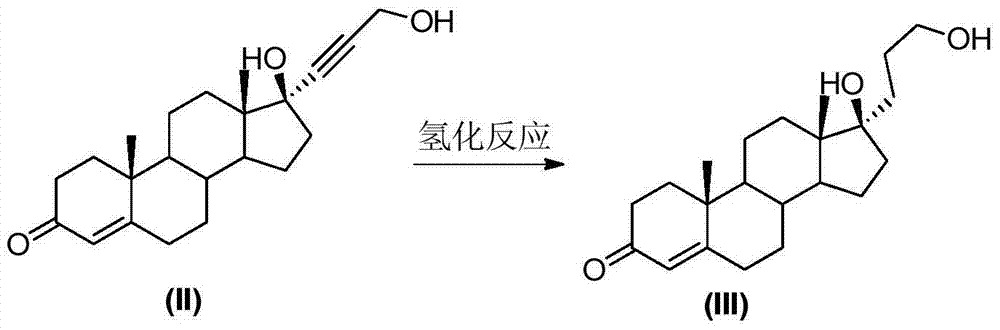
Patents
Literature
61 results about "4-Androstenedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
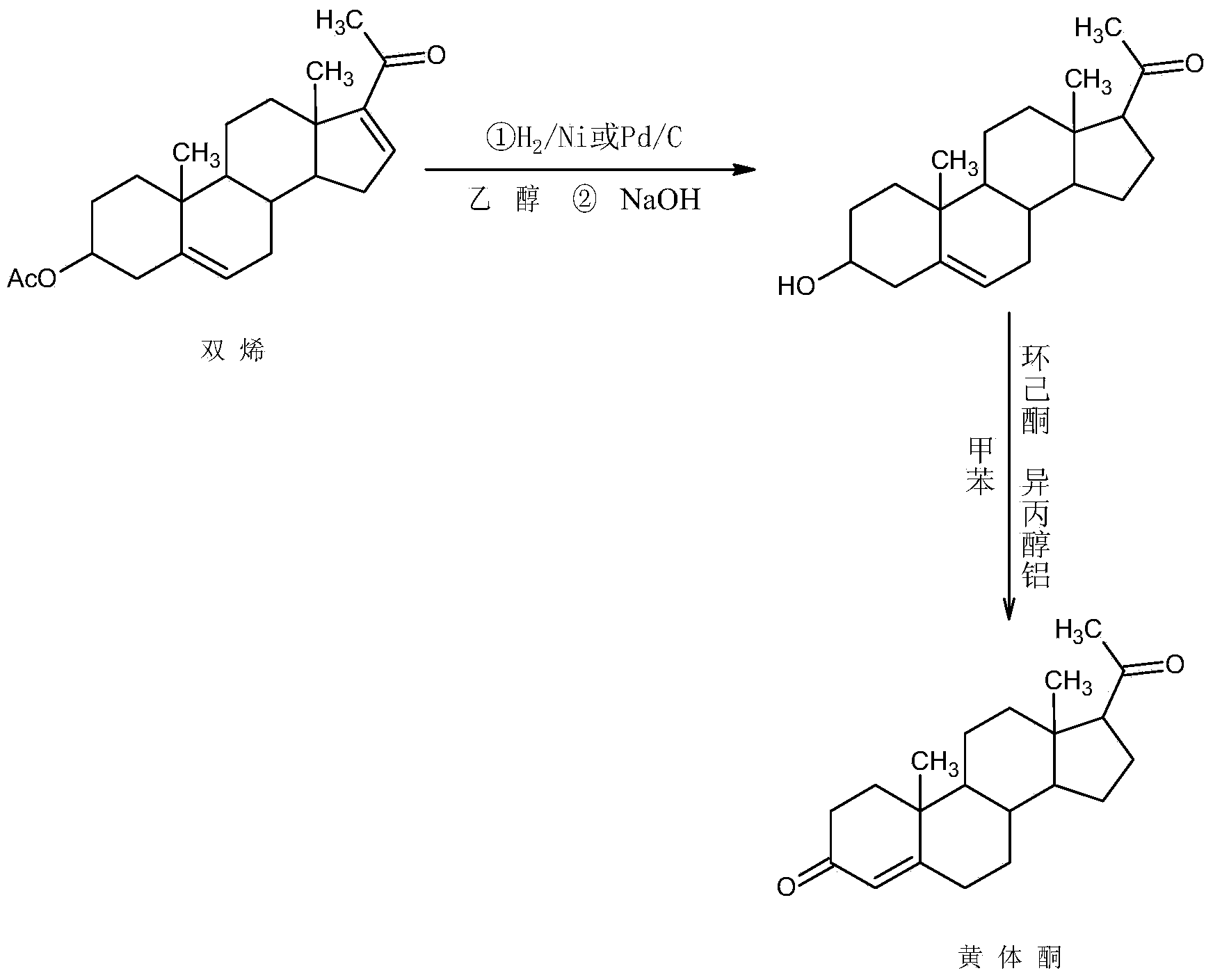
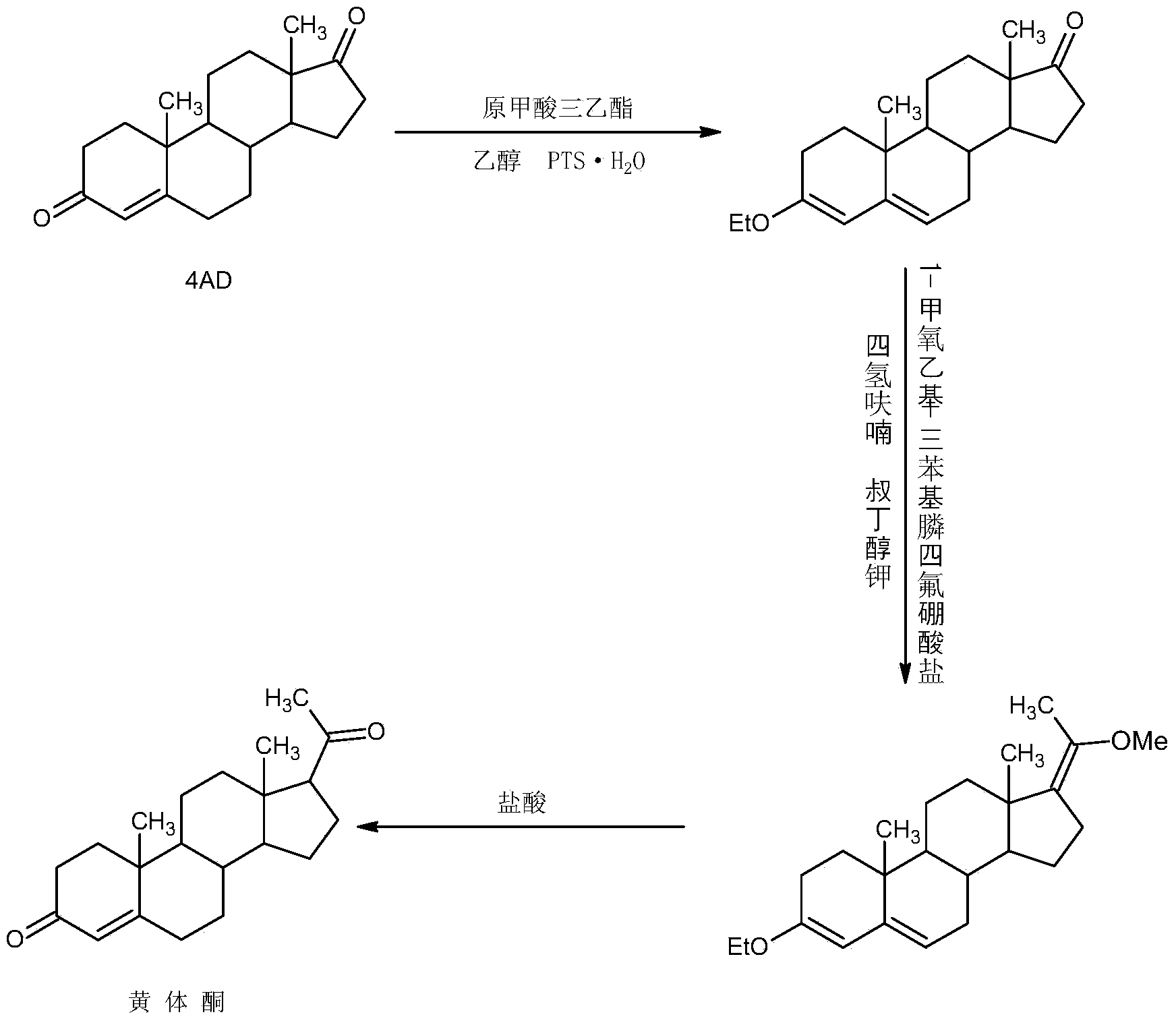
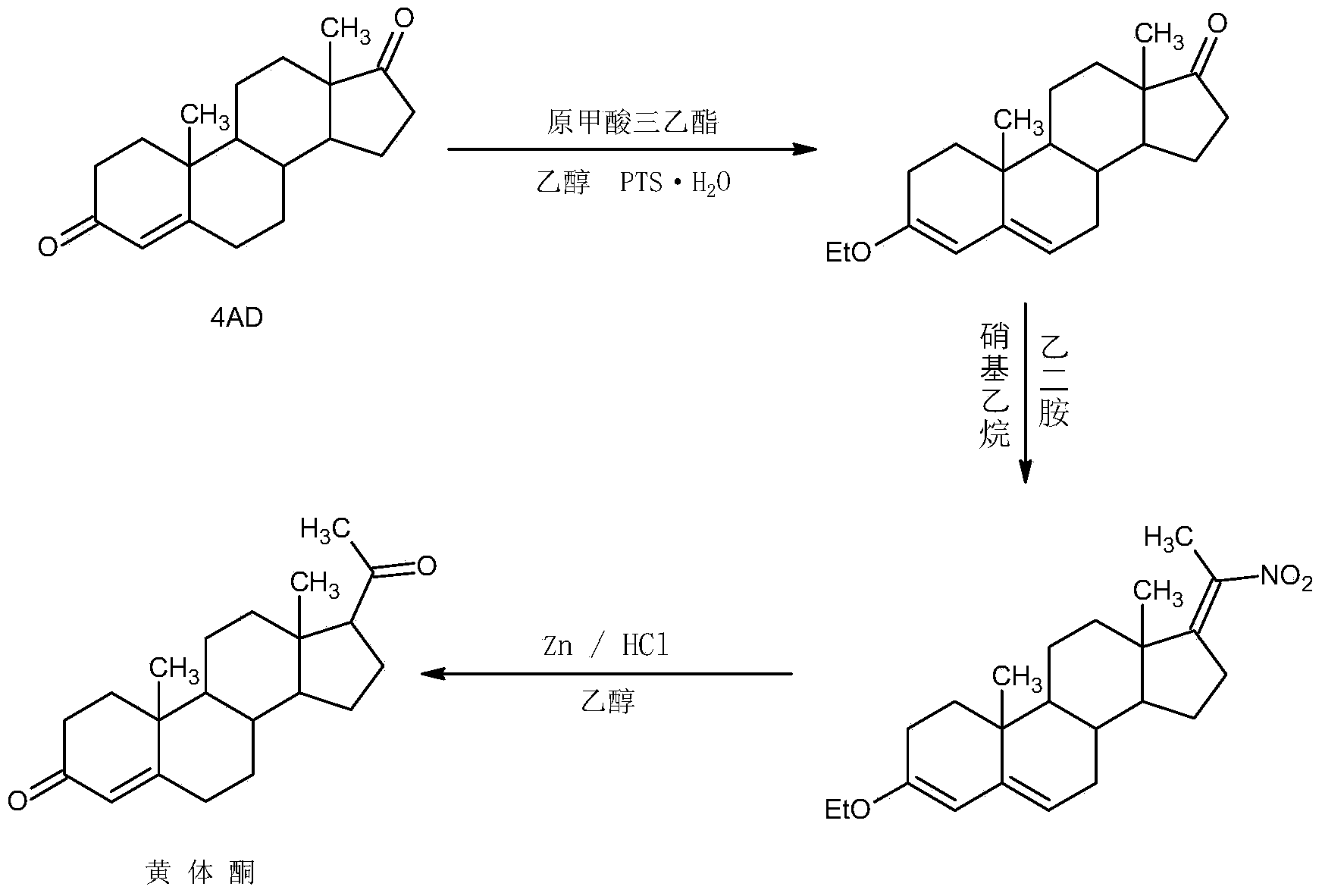
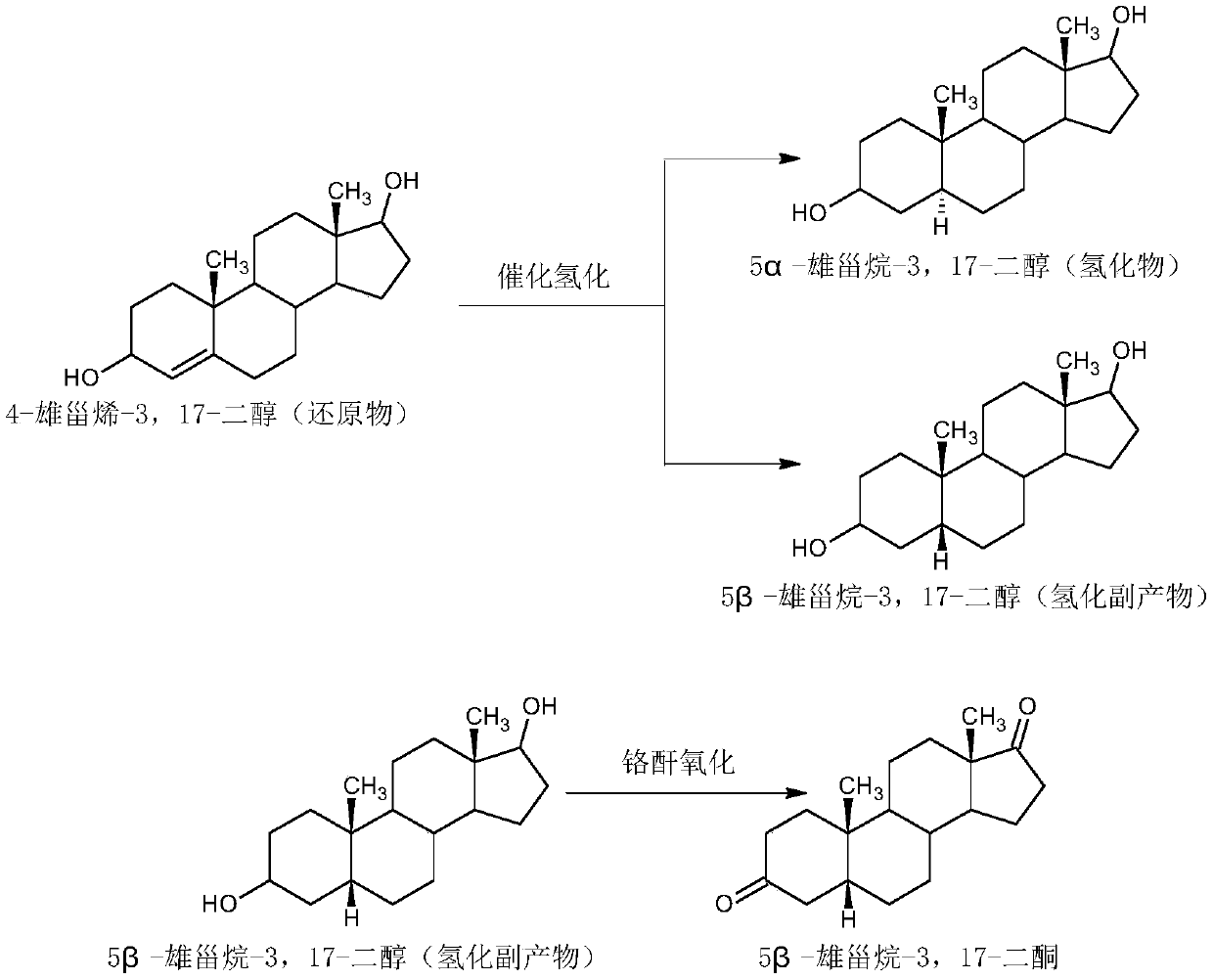
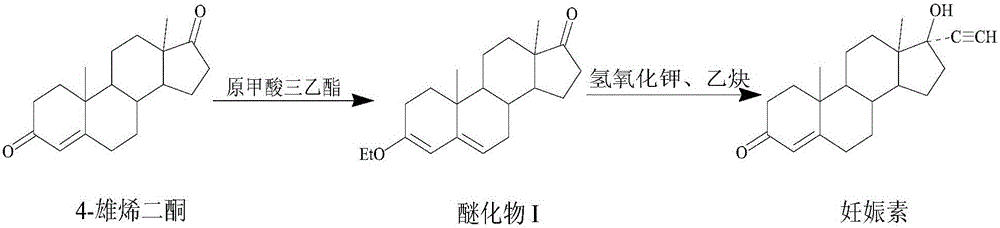
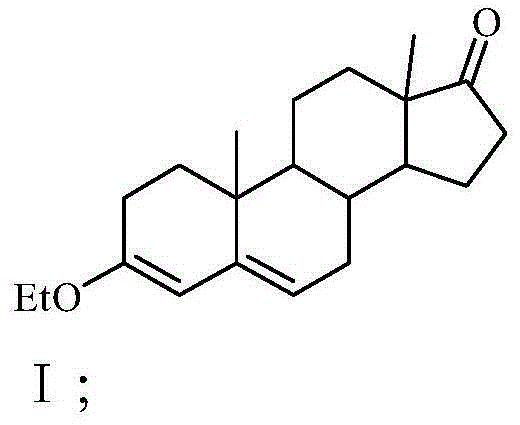
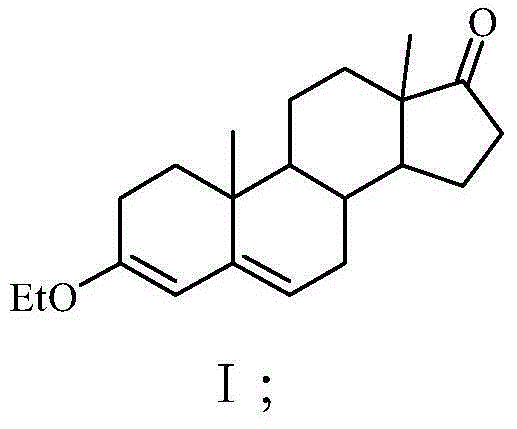
Preparation method for progestin
ActiveCN104262442AWide variety of sourcesProcess economy and environmental protectionSteroidsEthylenediamineKetone
The invention relates to a preparation method for progestin. 4-androstenedione is used as a raw material. The preparation method comprises the following steps: A, etherate is synthetized, wherein the 4-androstenedione and triethyl orthoformate perform an acid catalyzed reaction in organic solvents of dichloromethane, low-carbon alcohol and the like to obtain the etherate 3-ethoxy-androstane-3, 5-diolefin-17-ketone; B, a nitro substance is synthetized, wherein the etherate in the organic solvents and nitroethane perform 17-bit addition under the catalysis of ethylenediamine to obtain the nitro substance 3-ethoxy-20-nitro-pregnane-3, 5, 17 (20)-triene; and C, the progestin is synthetized, wherein the nitro substance is reduced by zinc powder in organic solvents of acetic acids, low-carbon alcohol and the like, acid hydrolysis is performed, so that semi-finished products of the progestin are obtained, the semi-finished products of the progestin are decolored and refined by alcohol and activated carbon to obtain the progestin, the content of HPLC is more than 99.5%, the melting point is 128-131 DEG C, and the total yield of synthetized weight is 83-87%. When the method disclosed by the invention is used for producing the progestin, the yield is high, the degree of purity is good, the quality is stable, the solvent recovering rate is high, and the method is economic and environment-friendly.
Owner:HUNAN KEREY BIOTECH
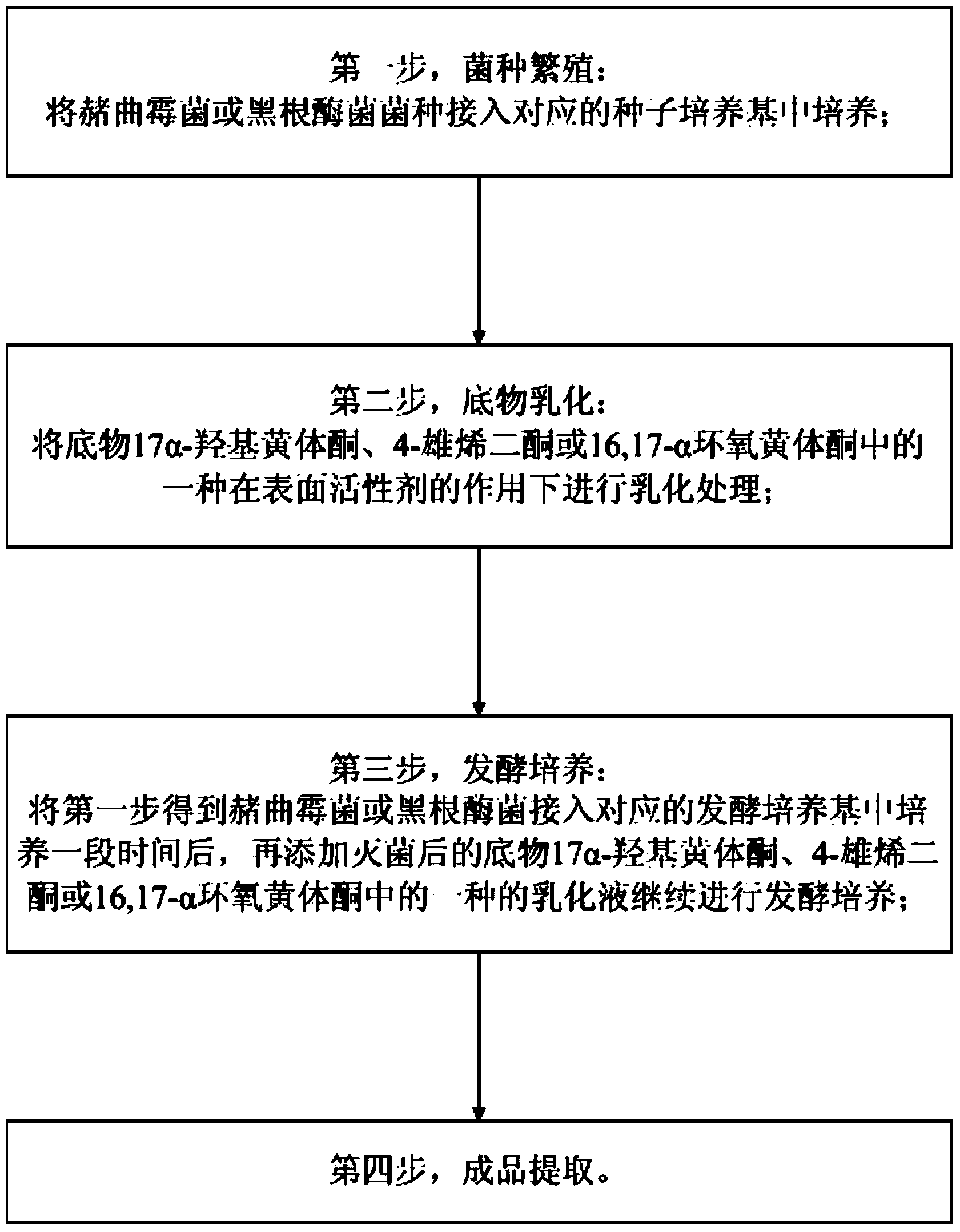
Preparation method for hydroxylation of 11 alpha of important intermediate of steroidal hormone substance
InactiveCN103966299AIncrease profitShorten the production cycleMicroorganism based processesFermentationEpoxyMicrobial transformation
The invention provides a preparation method for hydroxylation of 11 alpha of an important intermediate of a steroidal hormone substance, and aims to solve the problems that the conversion rate is low and the environment is polluted when a microorganism is used for converting steroidal C11 alpha for hydroxylation in the prior art. The preparation method comprises the following steps: strain breeding, wherein a strain of ochratoxin or rhizopus nigricans is inoculated to a corresponding seed medium for cultivation; substrate emulsification, wherein a substrate selected from one of 17-alpha hydroxyl progesterone, 4-androstenedione or 16,17-alpha epoxy progesterone is subjected to emulsification treatment under the action of a surfactant; fermented cultivation, wherein the ochratoxin or rhizopus nigricans is inoculated to a fermented medium to be cultivated for a period of time, and then one of emulsion liquids of sterilized 17-alpha hydroxyl progesterone, 4-androstenedione or 16,17-alpha epoxy progesterone is added for performing continuous fermented cultivation; extracting a finished product. The method has the advantages of high conversion rate, little pollution, environmental protection, low pressure and the like.
Owner:HEBEI ZHONGSHENG BIOTECH
Method for preparing progesterone
The invention relates to a method for preparing progesterone. According to the method, 4-androstenedione is used as raw materials, and hydroxyl cyanation, dehydration, protection, hydrogenation and addition hydrolysis reaction are carried out in sequence, and the progesterone is obtained. The 4-androstenedione which has the price advantage is used as the raw materials, the reaction condition is easy to control, post-processing is easy, and yield is high.
Owner:ZHEJIANG SHENZHOU PHARMA
Method for preparing dehydroepiandrosterone through chemical-enzyme method
The invention discloses a method for preparing dehydroepiandrosterone through a chemical-enzyme method. The method comprises that dehydroepiandrosterone is prepared from 4-androstenedione as an initial substrate orderly through a chemical method and a biological method. In preparation, the two reaction processes are optimized. In the chemical method-based 5-androstenedione preparation process, a reaction solution is added into an aqueous solution with sodium ascorbate and acetic acid so that reaction conditions are mild. In the second biological method-based dehydroepiandrosterone preparation process, a ketoreductase is used as a catalyst so that the product yield and purity are improved. In the whole reaction processes, use amounts of a coenzyme and potassium tert-butoxide are low and a high practical value is obtained.
Owner:ENZYMEWORKS
High-yield simple preparation method of 17alpha-hydroxy progesterone
The invention relates to a high-yield simple preparation method of 17alpha-hydroxy progesterone. With 4-androstenedione as an initial raw material, the method comprises the following steps: performing a cyanogen alcoholization addition reaction between the 17-site carbonyl of 4-androstenedione and acetone hydrogen alcohol to obtain 17-alpha hydroxyl-17-beta cyanoandrostane-4-ene-3-one; performing a ketal protection reaction of the C3-site carbonyl to obtain a ketal product; and performing a direct methylation reaction between the ketal product and zinc chloride methane, and hydrolyzing to obtain 17alpha-hydroxy progesterone. The method provided by the invention has the advantages of short process, high yield, high product purity, mild reaction conditions, low cost and low energy consumption and is particularly suitable for industrial production.
Owner:山东众诚生物医药股份有限公司
Method of extracting 4- androstenedione from plant sterol fermentation liquor
The invention discloses a method of extracting 4-androstenedione from plant sterol fermentation liquor. Particularly, 4-androstenedione crude products (I) and unreacted plant sterol are extracted from mycelium and slag charges which are separated from the fermentation liquor and soaked by acetone; the 4-androstenedione crude products (I) are dissolved and crystallized by acetone aqueous solution with a concentration of 50% so that crude products (II) are obtained; and the crude products (II) are recrystallized by alcohol so that pure 4-androstenedione is obtained, wherein the ratio of the alcohol to water is 1:10, and the unreacted plant sterol is recrystallized by the alcohol so that recovered plant sterol is obtained, wherein the ratio of the alcohol to water is 1:10. The method of extracting the 4-androstenedione from the plant sterol fermentation liquor is good in extraction effect, high in product purity, easy to operate, few in number of types of solvents used, capable of reducing environmental pollution, low in cost, short in process route and suitable for industrialized production. The unconverted plant sterol can be recovered and recycled.
Owner:SHANDONG DONGYAO PHARMACEUTICAL CO LTD
Preparation method of stanolone
InactiveCN106496297AWide variety of sourcesReduce manufacturing costEstrane derivativesDouble bondHydrolysis
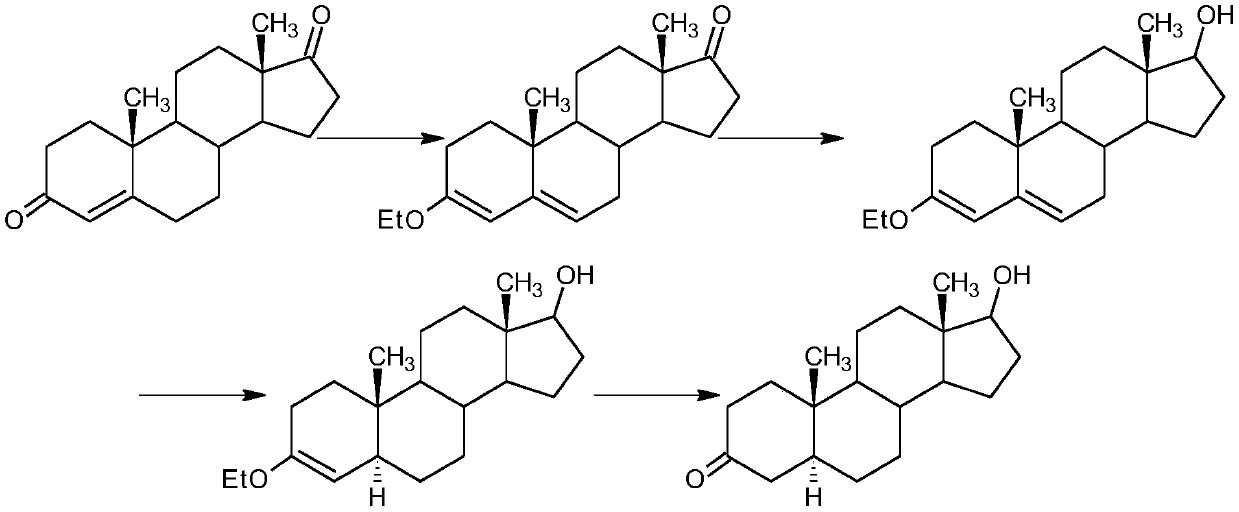
A preparation method of stanolone comprises the following steps: with 4-androstenedione (shortened as 4AD) as a raw material, firstly performing an acid-catalyzed reaction on the 4AD and triethyl orthoformate in an organic solvent to obtain etherate 3-ethoxy-androst-3,5-diene-17-one; then, adding the etherate into the organic solvent, adding metal borohydride as a reducing agent, and reducing a 17-one group in a molecule of the etherate into a hydroxyl group to obtain the 3-ethoxy-androst-3,5-diene-17-ol; then, dissolving a reduced product into the organic solvent, adding a hydrogenation reaction catalyst, selectively catalyzing a 5-position double bond in the molecule of the reduced product to obtain hydride 3-ethoxy-androst-3-ene-17-ol; finally, performing acid-catalyzed hydrolysis on the hydride in the organic solvent to obtain the stanolone. Compared with the conventional production method, the preparation method provided by the invention has the advantages as follows: the raw material source is wide, the synthesis route is short, the preparation method is simple and environment-friendly in process, the product yield is high, the preparation method is economical and environment-friendly, the production cost is reduced by 35-40%, and the preparation method is very conductive to industrial production.
Owner:HUNAN KEREY BIOTECH
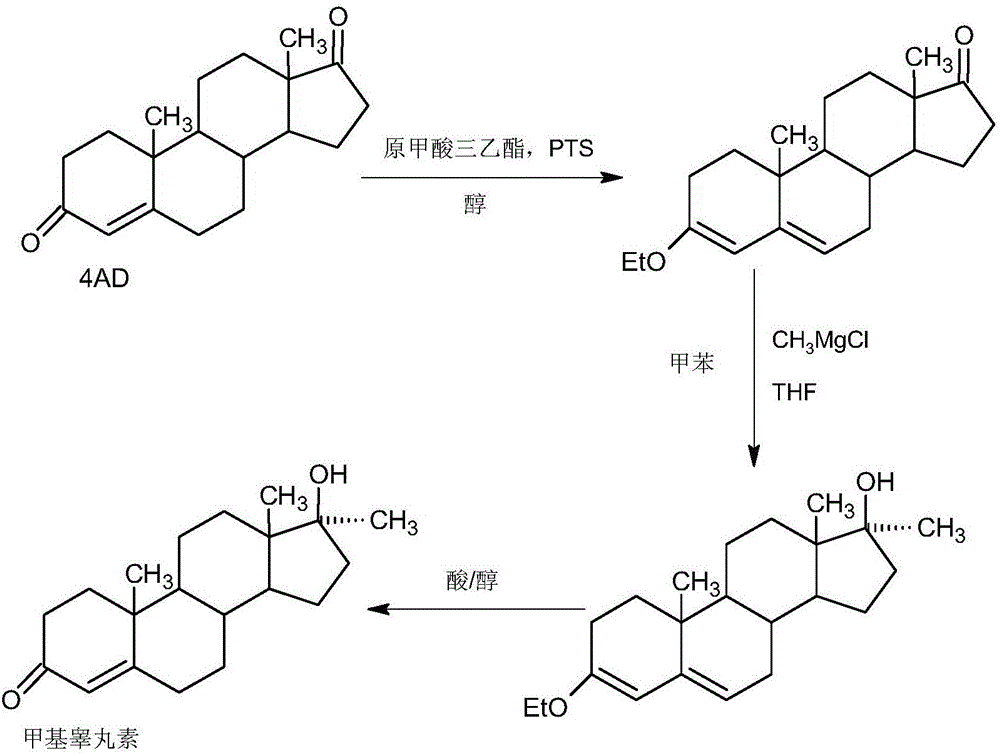
Preparation method of methyltestosterone
InactiveCN106397520AWide variety of sourcesProcess economy and environmental protectionSteroidsGrignard reagentKetone
The invention provides a preparation method of methyltestosterone. 4AD short for 4-androstenedione is taken as a raw material, and etherate is synthesized firstly as follows: 4AD and triethyl orthoformate are subjected to an acid catalyzed reaction in a low-carbon alcohol organic solvent, and 3-ethoxy-androst-3,5-diene-17-one as the etherate is obtained; then a Grignard product is synthesized as follows: a Grignard reagent methyl magnesium halide and the etherate are placed in an organic solvent, the 17-position ketone group of the etherate and the Grignard reagent are subjected to addition, and the Grignard product 3-ethoxy-17a-methyl-androst-3,5-diene-17-ol is obtained through hydrolysis; then the Grignard product is subjected to an acid catalyzed hydrolysis in an organic solvent, and crude methyltestosterone is obtained; the crude methyltestosterone is decolorized by activated carbon in C4-below low-carbon alcohol and recrystallized, the methyltestosterone is obtained, HPLC content is 99.0%-99.5%, and the total yield of synthesis weight is 75%-78%. According to the method, the raw materials are widely sourced, the process is simple and convenient to operate, the product yield is high, the purity is good, the solvent recovery rate is high in reaction and technological processing, and the method is economical and environment-friendly.
Owner:HUNAN KEREY BIOTECH
Method for preparing 19-demethyl-4-androstenedione
The invention discloses a method for preparing 19-demthyl-4-androstenedione, which comprises the steps of: based on estrone as a raw material, sequentially carrying out etherealization reaction, ketalation, Birch reducing reaction and hydrolysis reaction to obtain19-demethyl-4-androstenedione, wherein the reaction formula is shown in the specification. According to the method provided by the invention, the traditional phenolic group methyl etherealization process is improved, methyl carbonate replaces traditional high-toxicity carcinogenic etherealization reagents dimethyl sulfate and iodomethane, and inexpensive potassium carbonate is used as a base, so that the production cost is lowered, the method is environment-friendly, the yield is high, and the method is suitable for scaled industrial production.
Owner:ZHEJIANG XIANJU PHARMA
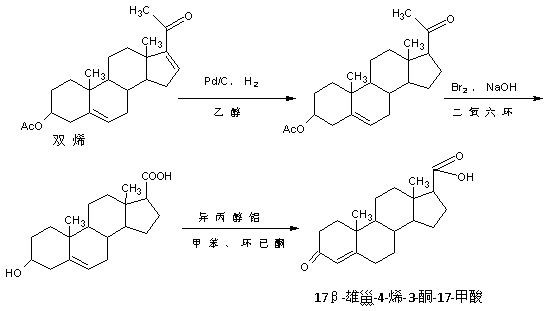
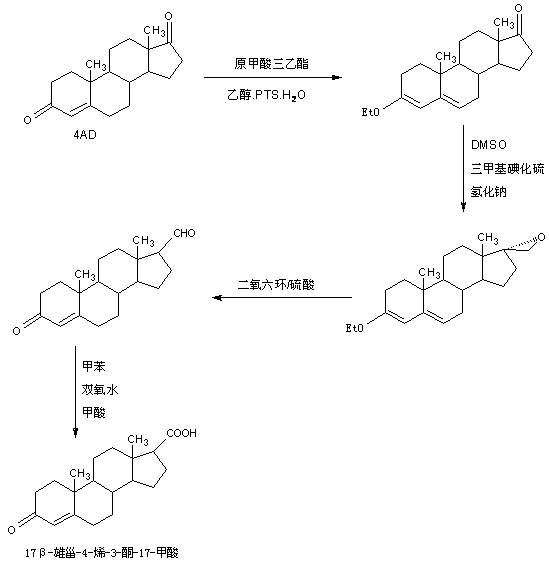
Preparation method of 17 beta-androst-4-ene-3-one-17-carboxylic acid
ActiveCN107629101ARaw materials are cheap and easy to getThe process is simple and environmentally friendlySteroidsCatalytic oxidationCarboxylic acid
The invention discloses a preparation method of 17 beta-androst-4-ene-3-one-17-carboxylic acid. The preparation method comprises that 4-androstenedione (called as 4AD for short) as a raw material andtriethyl orthoformate undergo a reaction in an organic solvent under acid catalysis, the reaction product is after-treated to form an etherate, the etherate is dissolved in an organic solvent and undergoes a reaction with trimethylsulfonium iodide under strong base catalysis, the product is after-treated to form an epoxide, the epoxide is dissolved in an organic solvent and then is subjected to rearrangement under acid catalysis to form an aldehyde, and aldehyde is dissolved in an organic solvent and undergoes a catalytic oxidation reaction with hydrogen peroxide acid to produce 17 beta-androst-4-ene-3-one-17-carboxylic acid. The 17 beta-androst-4-ene-3-one-17-carboxylic acid has a melting point of 244-246 DEG C, HPLC content of 99.0% or more and a reaction weight total yield of 70-72%. Compared with the traditional method, the preparation method utilizes cheap and easily available raw materials, has simple and environmental friendly processes and a high synthesis yield, produces highquality products, reduces a cost by 30-40% and is conducive to industrial production.
Owner:HUNAN KEREY BIOTECH
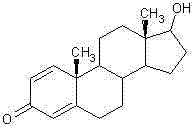
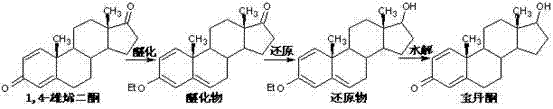
Method for preparing boldenone through selective reduction
The invention provides a method for preparing boldenone through selective reduction. 1,4-androstenedione is used as a main raw material, the charging sequence is changed, the main raw materials are treated in advance, the charging mode of the main raw materials is changed, and the keto selective reduction reaction is carried out, thus, the boldenone is prepared. The method solves the problems that the existing boldenone preparation process has more steps, long period and high production cost. According to the preparation method, the production procedures are reduced, the production period is shortened, the operation is simple, the cost is low and the method is suitable for industrial production.
Owner:HUAZHONG PHARMA
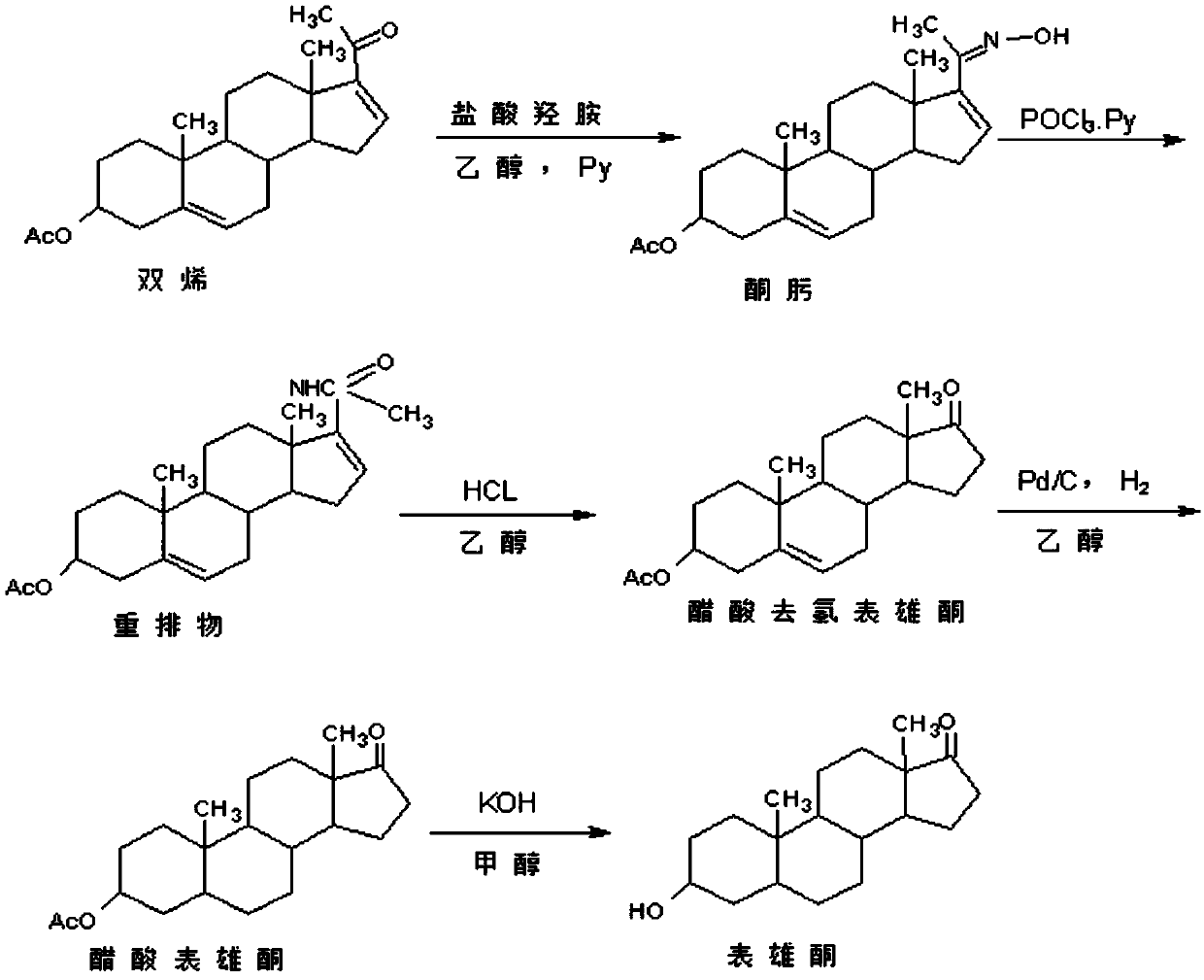
Method for preparing epiandrosterone
The invention discloses a method for preparing epiandrosterone. 4-androstenedione (I) is used as a raw material to prepare the epiandrosterone. The reaction formula is shown in the description. The low-cost 4-androstenedione is used as the raw material for the first time, and the epiandrosterone is obtained through synthesis on the mild reaction conditions, so that the production cost is greatly lowered, and the method is suitable for large-scale industrial production.
Owner:仙琚(嘉兴)医药科技有限公司 +1
Synthesis method of spironolactone intermediate canrenone
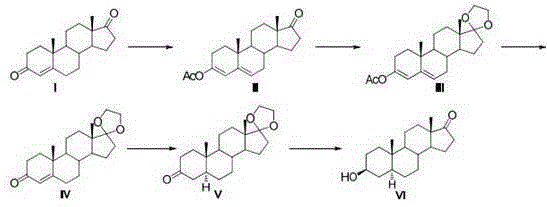
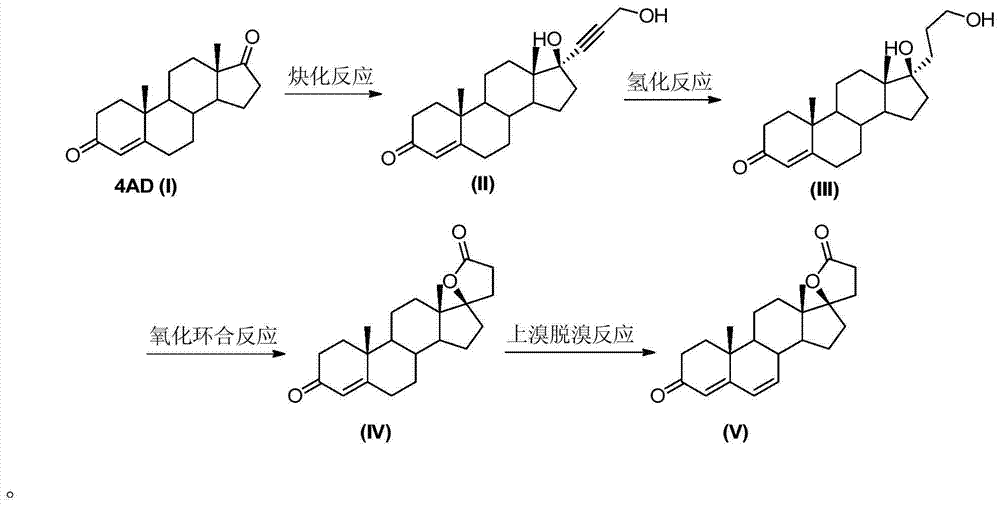
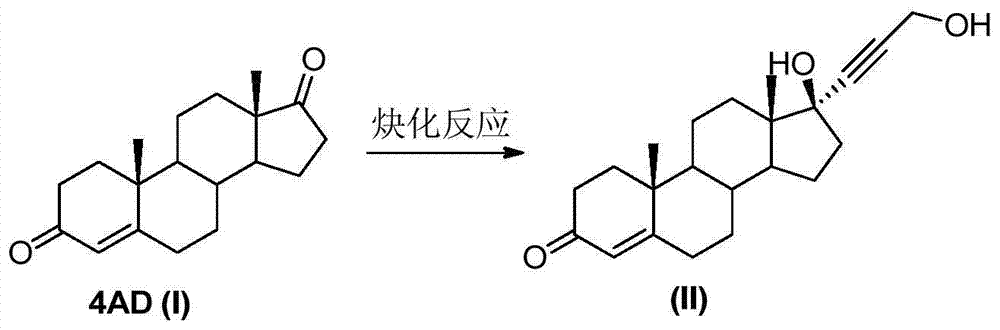
The invention relates to a synthesis method of a chemical medicine, and concretely relates to a synthesis method of a spironolactone intermediate canrenone. The method comprises the following steps: carrying out an ethynylation reaction on a compound I 4-androstenedione (4AD), hydrogenating, carrying out an oxidation cyclization reaction, and carrying out a bromization and debromination reaction to obtain the compound V canrenone, and the above reaction route is shown in the specification. A synthesis method of the structure of an important 21,17-carboxy lactone spiro ring adopted in the invention is different from previous process modes, and is concise and efficient. The method has the characteristics of high yield, good selectivity, low cost, mild reactions, suitableness for industrialization, stability and easy realization.
Owner:ZHEJIANG SHENZHOU PHARMA
Preparation method for dehydroepiandrosterone, and enzyme for preparation thereof
InactiveCN109312382AHigh yieldSimple processMicroorganismsMicroorganism based processesSodium ascorbate4-Androstenedione
A preparation method for dehydroepiandrosterone, comprising: in a protective atmosphere, adding potassium tert-butoxide to tert-butanol, stirring evenly, adding 4-androstenedione to obtain a mixture,and adding the mixture dropwise to a sodium ascorbate-containing acetic acid solution for a reaction to obtain 5-androstenedione; dissolving the 5-androstenedione in an organic solvent, adding a ketone reductase, a glucose dehydrogenase, glucose and a redox coenzyme to obtain a mixture, controlling the pH of the mixture to be 6.0-6.3, stirring and reacting for 1-6 hours at 22-26 DEG C to obtain areaction solution, and performing separation and purification on the reaction solution to obtain dehydroepiandrosterone, the ketone reductase and the glucose dehydrogenase being coexpressed by a microbial strain and added in the form of a crude enzyme solution. The synthesis process of the preparation method has few steps, simple operations, high yields and low costs, and may be widely applied toindustrial scale production. Also provided is an enzyme for preparation.
Owner:BONTAC BIO ENG SHENZHEN
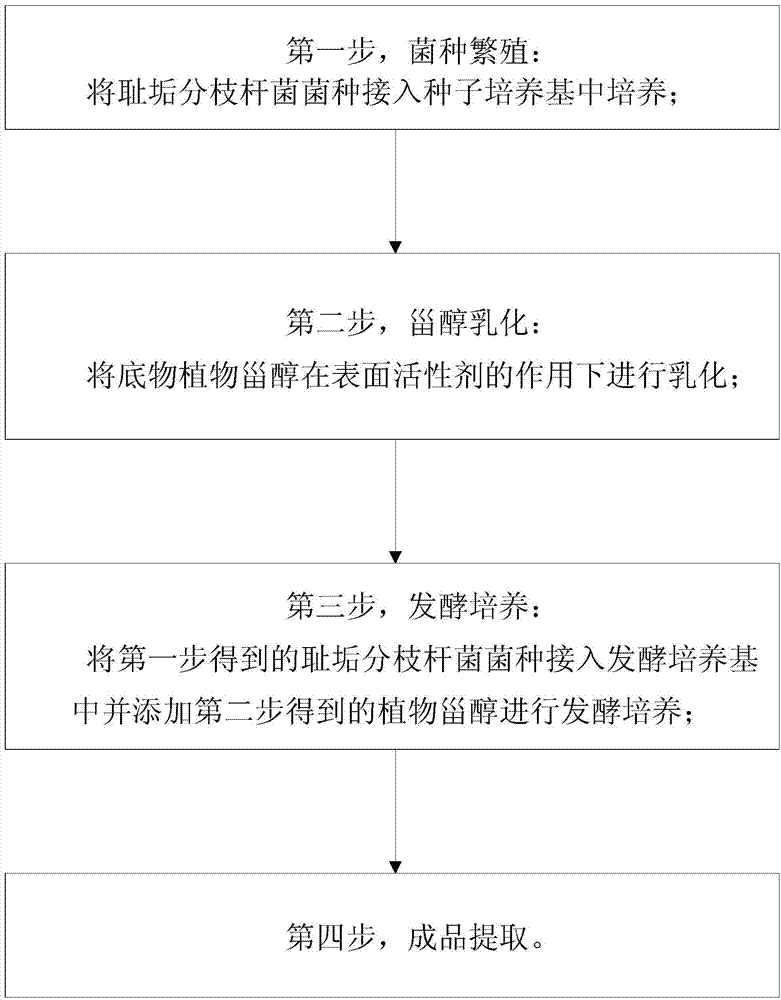
Method of preparing 4-androstenedione by fermentatively degrading phytosterol by virtue of single water phase system
InactiveCN103923966AIncrease profitReduce typesMicroorganism based processesFermentationSterolMycobacterium smegmatis
The invention provides a method of preparing 4-androstenedione by fermentatively degrading phytosterol by virtue of a single water phase system. The method comprises the following step: 1, strain cultivation, namely inoculating a mycobacterium smegmatis stain into a seed medium and cultivating; 2, sterol emulsification, namely emulsifying the phytosterol as a substrate under the action of a surfactant; 3, fermentation cultivation, namely inoculating the mycobacterium smegmatis stain obtained in the first step in a fermentation medium, adding the phytosterol obtained in the second step and carrying out fermentation cultivation; 4, finished product extraction. According to the method of preparing the 4-androstenedione by fermentatively degrading the phytosterol by virtue of the single water phase system, an emulsifier is added, so that the yield of 4-androstenedione is increased, and the extraction technology of the 4-androstenedione is also simplified; the cost is low, the pollution is little, and the environmental protection pressure is weak.
Owner:HEBEI ZHONGSHENG BIOTECH
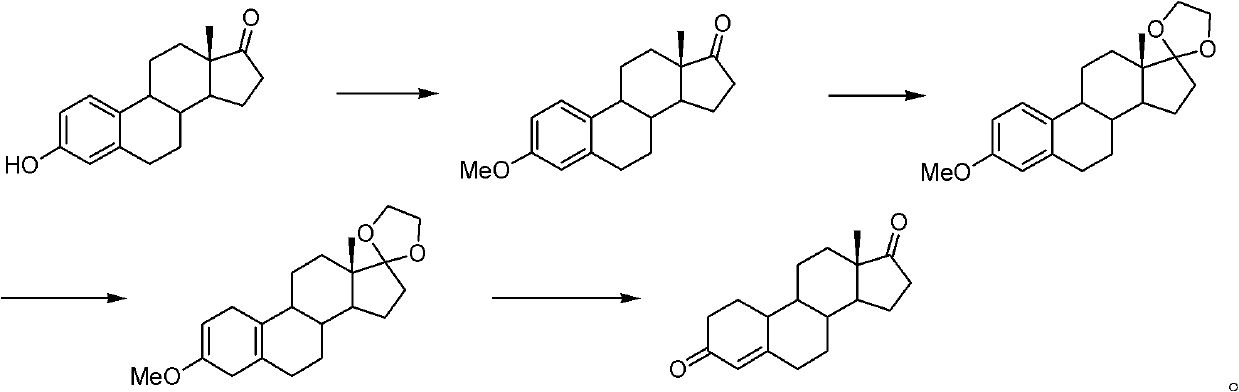
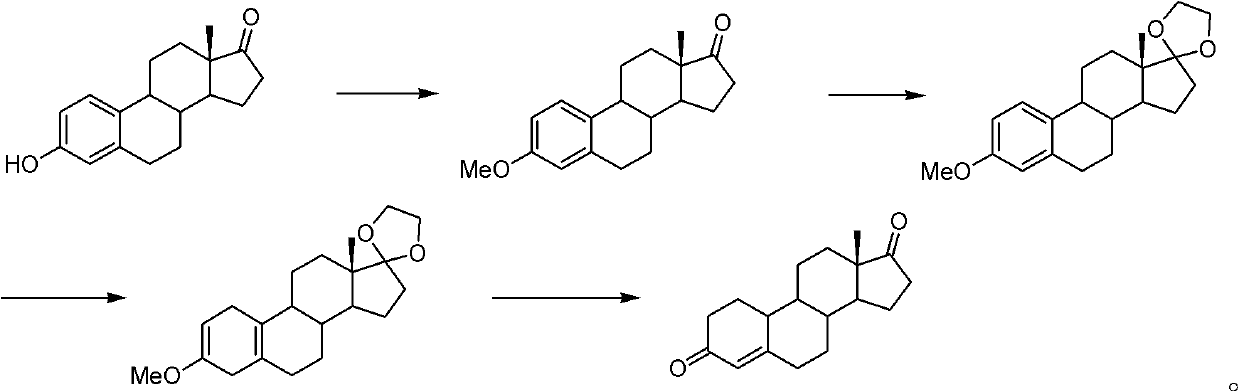
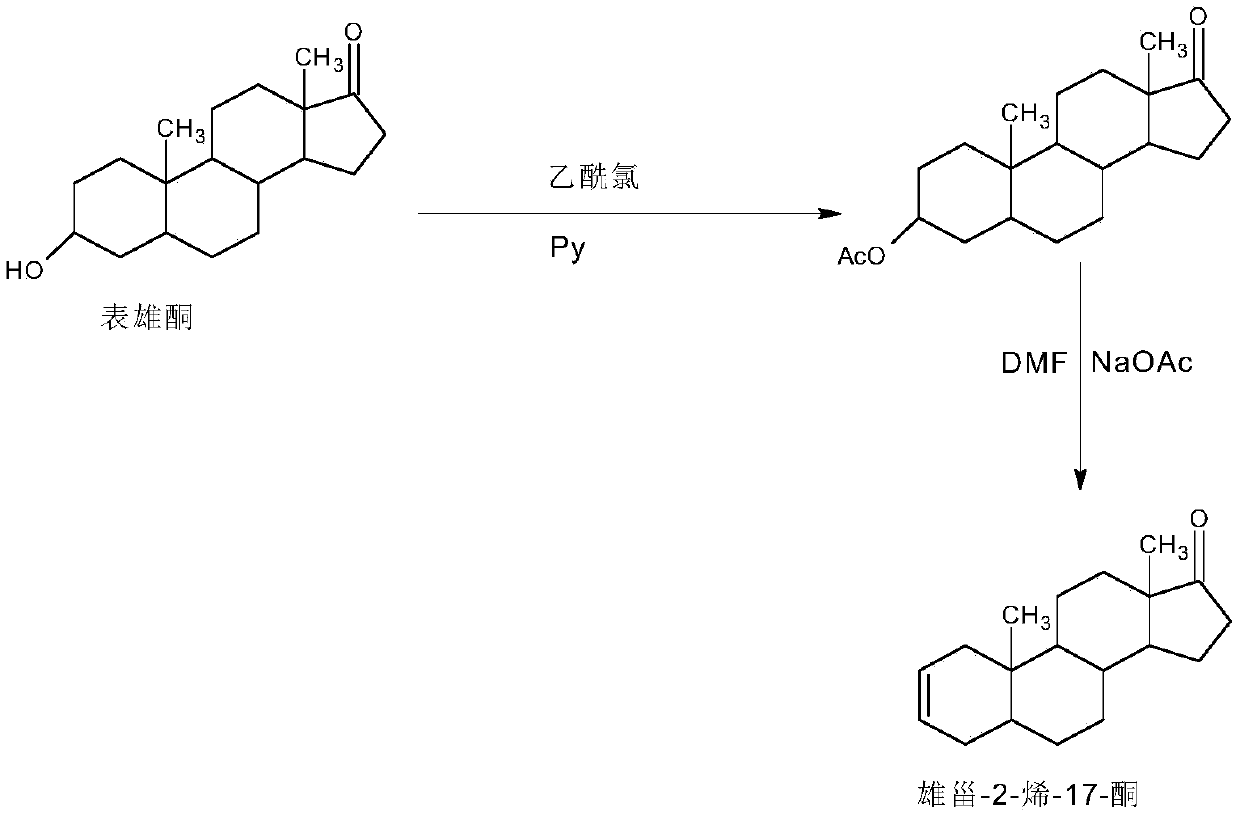
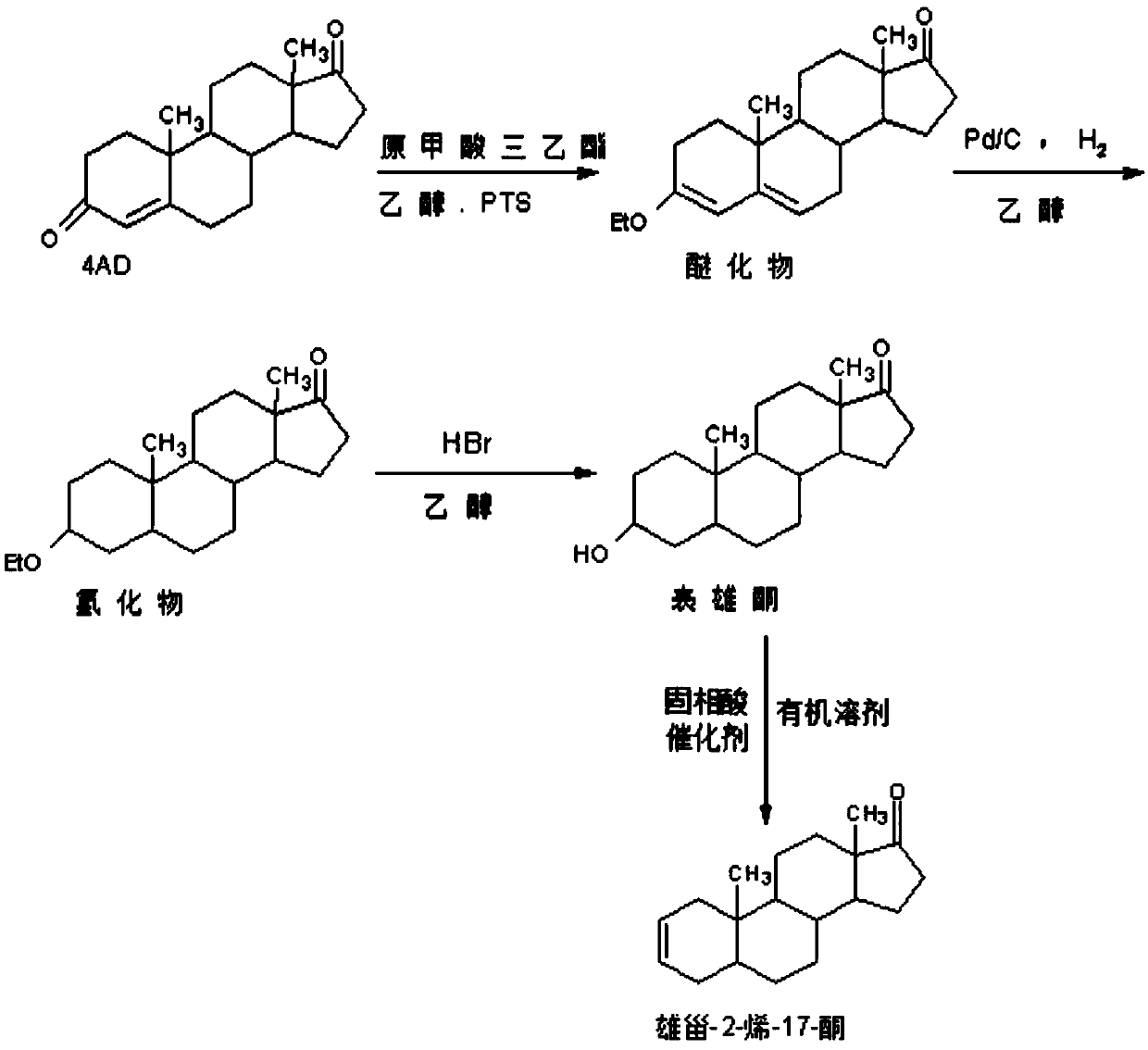
Preparation method of androst-2-en-17-one
The invention discloses a preparation method of androst-2-en-17-one. The method comprises the steps that 4-androstenedione is adopted as a raw material, three-step reactions of a protective reaction,a reduction reaction and a deprotection reaction are adopted for synthesizing epiandrosterone, in an organic solvent, a carrier solid phase acid catalyst soaked with acid is used for catalyzing, epiandrosterone dehydration is performed to eliminate 3-bit hydroxyls in molecules, and a target product is obtained. 4-androstenedione obtained by phytosterol extracted from a soybean oil deodorization distillate through the modern fermentation technology is adopted as a raw material; 4-androstenedione is subjected to the three-step reactions to synthesize a key intermediate epiandrosterone, and raw materials are abundant in source and low in price. The epiandrosterone is used for preparing a target product, compared with a traditional method, a synthetic route is cut into a one-step reaction fromtwo-step reactions, the method is economical and environmentally friendly, and production and operation are easier. By adopting the carrier solid phase acid catalyst soaked with acid for catalyzing,an oligomer makes indirect contact with the acid catalyst, the reactions are mild and stable, the dehydration elimination reactions are performed in the thermodynamics stable 2,3-bit directions, and the reaction selectivity is good.
Owner:HUNAN KEREY BIOTECH
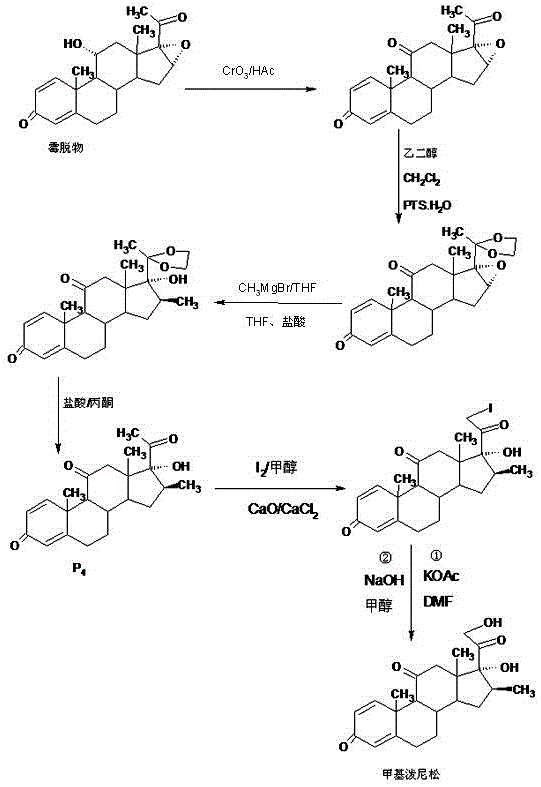
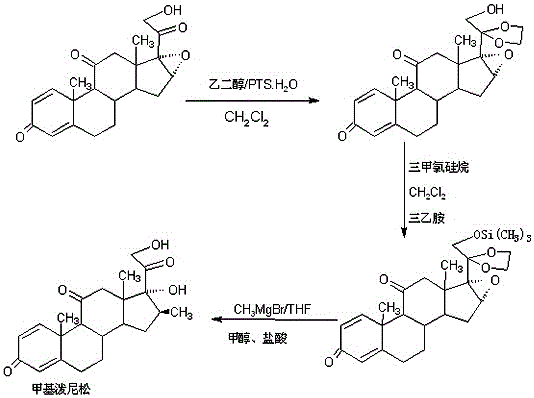
Preparation method of methylprednisone
ActiveCN106518944AWide variety of sourcesProcess economy and environmental protectionSteroidsGrignard reagentStrong acids
The invention relates to a preparation method of methylprednisone. The method comprises the following steps: carrying out acid catalyzed reaction on 16(17)a-epoxy prednisone prepared from 4-androstenedione (4AD for short) and ethanediol in an organic solvent at 10-50 DEG C to obtain a ketal substance 20-ketal-16(17)a-epoxy prednisone; carrying out alkali catalyzed reaction on the ketal substance and trimethylchlorosilane in an organic solvent to obtain a silyl ether substance 21-methylsilyl-ether-20-ketal-16(17)a-epoxy prednisone; and carrying out Grignard reaction on the silyl ether substance and a 2M methyl grignard reagent in an organic solvent, hydrolyzing the Grignard substance in strong acid to obtain the methylprednisone. The detection indicates that the HPLC (high performance liquid chromatography) content is 99.0% or above, the melting point is 228-237 DEG C, and the synthetic weight total yield is 80-85%. When being used for producing methylprednisone, the method has the advantages of wide raw material sources, economical and environment-friendly technique, simple production operation, short synthesis route, high synthesis yield and lower production cost (than the traditional method by 30-40%). The method is convenient for industrial production.
Owner:HUNAN KEREY BIOTECH
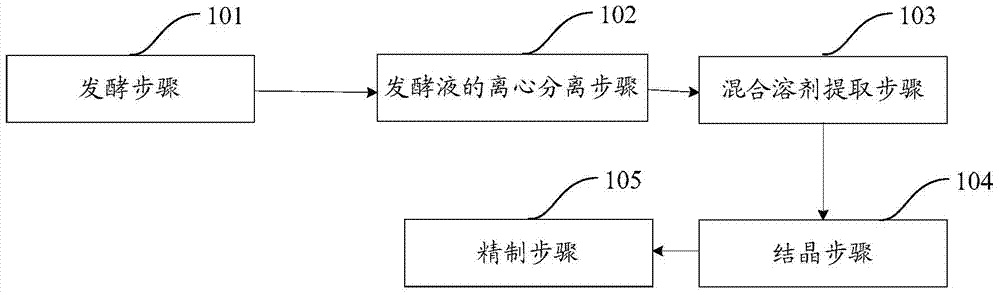
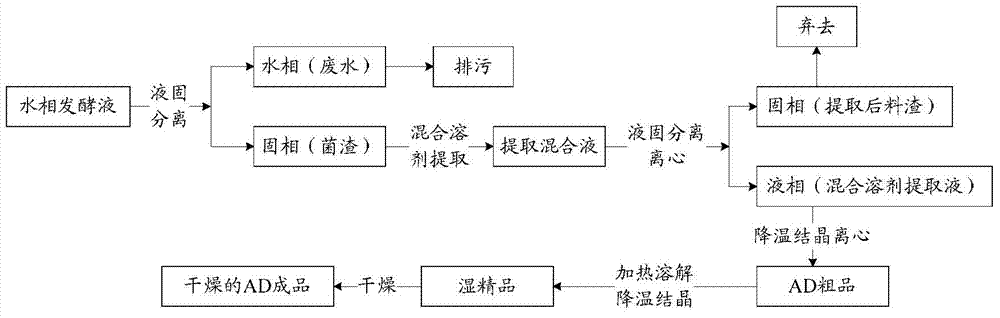
Method and process for extracting 4-androstenedione from phytosterol aqueous phase fermentation liquor
The invention provides a method and process for extracting 4-androstenedione from phytosterol aqueous phase fermentation liquor. The method comprises the following steps: performing microbial fermentation on phytosterol serving as a raw material to generate 4-androstenedione; performing liquid-solid separation on the fermentation liquor generated at the end of fermentation through a centrifugal machine, and extracting a wet solid material; adding a acetone-purified water mixed solvent into the wet solid material, continuously stirring for 60-90 minutes under the condition that the temperature is raised to 40-60 DEG C, and performing solid-liquid separation under the temperature condition of higher than 40 DEG C; crystallizing the solution obtained through solid-liquid separation; centrifuging the crystallized material to obtain a coarse product of the 4-androstenedione, and refining the coarse product to prepare the finished 4-androstenedione product. According to the method, the impurities in the 4-androstenedione product can be reduced, and the extracting and refining process is simplified.
Owner:SHANDONG SITO BIO TECHNOLOGY CO LTD
Preparation method of 19-demethylation-4-androstenedione
The invention discloses a preparation method of 19-demethylation-4-androstenedione and belongs to the technical field of medical intermediate processing. The preparation method comprises the following steps: (1) carrying out esterification reaction; (2) carrying out ketalation; (3) carrying out reduction reaction; (4) carrying out hydrolysis reaction; (5) carrying out esterification reaction; (6) carrying out addition reaction; (7) carrying out cyclization and hydrolysis reaction; (8) carrying out oxidation, dechloridation and ring-opening reaction; and (9) carrying out oxidation and decarboxylation reaction. The preparation method disclosed by the invention has the advantages that environmental pollution is small, usage amounts of a solvent and water are smaller, temperature sensitivity is low, control is easy, and yield is high; a chlorinating agent is adopted, so that reaction yield is increased; 1,3-dichloro-5,5-dimethylhydantoin is adopted, so that the reaction yield is further increased; and sodium hydrogen carbonate is adopted, so that final decarboxylic reaction yield is increased, and external standards and appearance of a product can be improved.
Owner:上海津力药业股份有限公司
Technology for refining 4-androstenedione from dehydroepiandrosterone mother solution
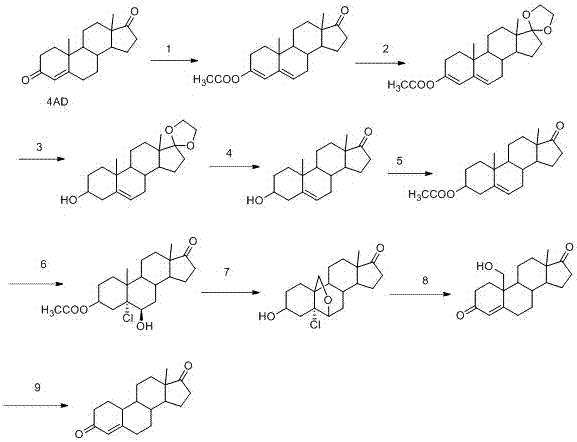
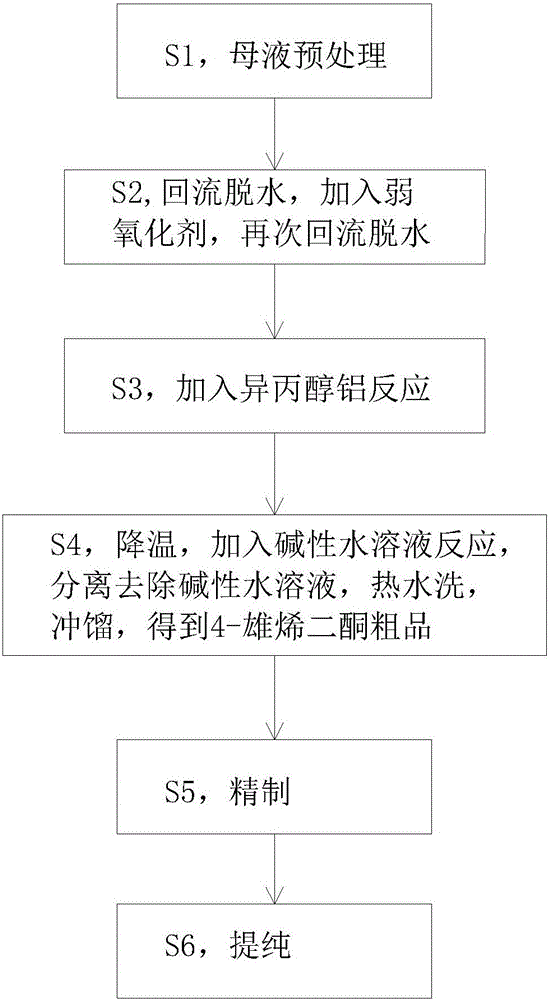
The invention discloses a technology for refining 4-androstenedione from a dehydroepiandrosterone mother solution. The technology comprises the steps that dehydroepiandrosterone is pretreated, reflux dehydration, addition of a weak oxidant, reflux dehydration and addition of aluminum isopropoxide for a reaction are carried out in sequence, an alkaline aqueous solution is added for a reaction, washing and distillation are carried out, and crude 4-androstenedione is obtained; then refining and purifying are carried out, and the 4-androstenedione product high in purity and yield is obtained.
Owner:湖北省丹江口开泰激素有限责任公司
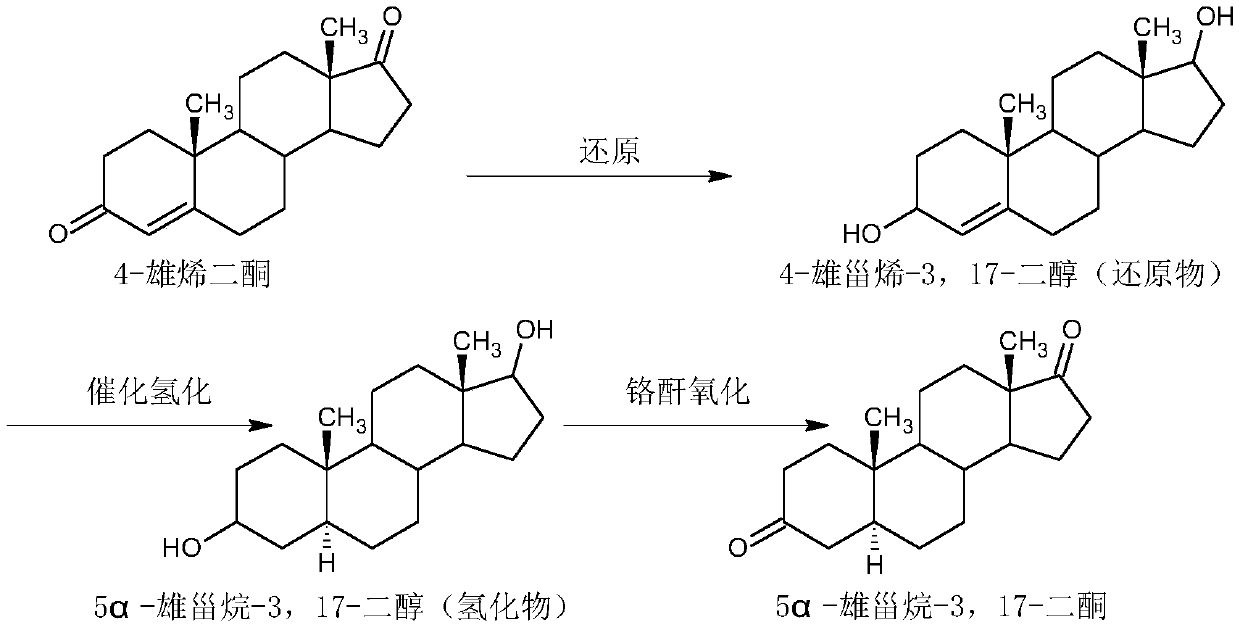
Preparation method of 5alpha-androstane-3,17-dione
The invention relates to a preparation method of 5alpha-androstane-3,17-dione. The method specifically comprises the following steps: 1, carrying out an etherification reaction on 4-androstenedione, triethyl orthoformate and anhydrous ethanol in the presence of an etherification catalyst, and performing post-treatment after the etherification reaction is completed in order to prepare a compoundwet 3-ethoxy-3,5-androstadien-17-one; 2, adding a the wet 3-ethoxy-3,5-androstadien-17-one into a methanol-dichloromethane mixed solvent, performing uniform stirring, adjusting the pH value of the obtained solution, carrying out a catalytic hydrogenation reaction under the action of a palladium-carbon catalyst, and filtering out the catalyst after the reaction is finished in order to obtain a 3-ethoxy-3-androstene-17-one solution; and 3, carrying out a hydrolysis reaction on the 3-ethoxy-3-androstene-17-tone solution and an acid, removing the solvent after the hydrolysis reaction is finished,and carrying out filtering, water washing and drying to obtain the 5alpha-androstane-3,17-dione. The method has the advantages of short process route, easily controlled production process, environmental friendliness, low production cost, and suitableness for industrial large-scale production.
Owner:HUAZHONG PHARMA
Synthetic method for ethisterone
InactiveCN105001294ASignificantly progressiveImprove the yield of etherification reactionSteroidsIsobutanolPotassium hydroxide
Owner:BAOJI KANGLE BIOTECH
Efficient breeding method of 4-androstenedione strains
InactiveCN105567599AImprove screening efficiencyIncrease the number of culturesBacteriaComponent separationBiotechnologySolvent
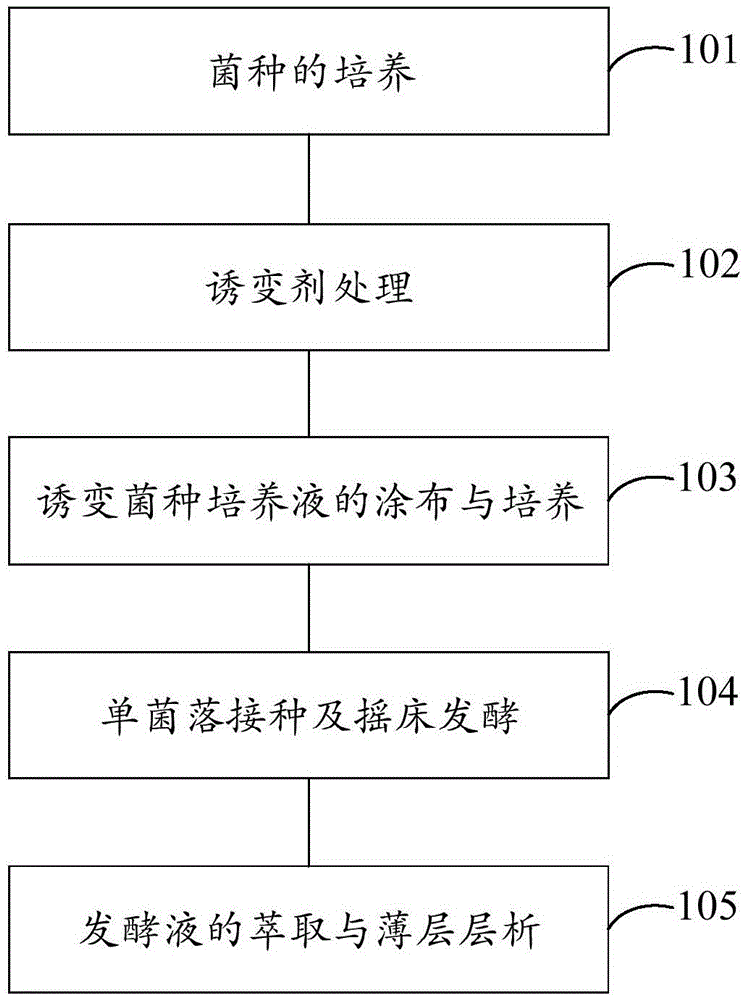
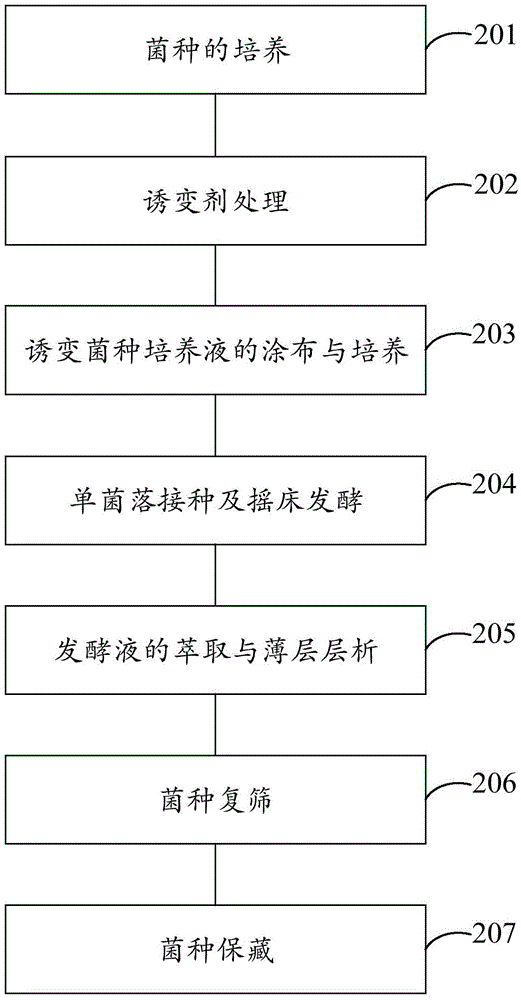
The invention provides an efficient breeding method of 4-androstenedione strains. The method includes the five steps of strain culturing, mutagenesis treatment, mutagenic strain culture solution coating and culturing, single colony inoculation and shaking table fermentation, and fermentation broth extraction and thin layer chromatography. By the adoption of the method, miniature fermentation tubes are used, thousands of miniature fermentation tubes can be cultured through one shaking table experiment, in this way, the number of cultured strains is increased per unit time, the culturing time for a preset number of strains is shortened, and then the efficiency of strain screening is improved. Meanwhile, because the miniature fermentation tubes are used, solvent can be directly added into the miniature fermentation tubes for extraction after fermentation is ended; in this way, the extraction steps are simplified, and operation time is shortened. In addition, the bi-phase thin layer chromatography method is adopted for replacing an efficient liquid phase method, an experimenter can detect hundreds of samples each day, in this way, the screening speed of the strains is increased, and the efficiency of strain screening is substantially improved.
Owner:SHANDONG SITO BIO TECHNOLOGY CO LTD
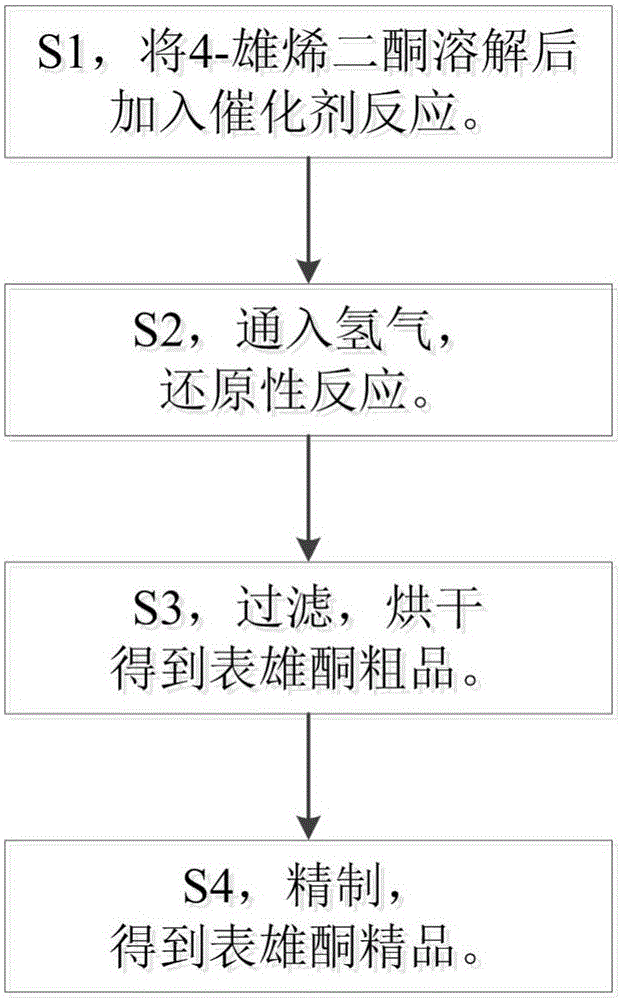
Novel technology for synthesizing epiandrosterone by adopting 4-androstenedione through selective hydrogenation reducing
The invention provides a novel technology for synthesizing epiandrosterone by adopting 4-androstenedione through selective hydrogenation reducing.Epiandrosterone is synthesized by adopting 4-androstenedione through selective hydrogenation reducing, therefore, the reaction path is greatly shortened, generation of an intermediate reaction by-product is prevented, and the product purity and yield are high; in addition, the reaction raw material consumption is lower, the cost is reduced, and the concept requirement for green production is met.
Owner:湖北省丹江口开泰激素有限责任公司
Preparation method for finasteride intermediate
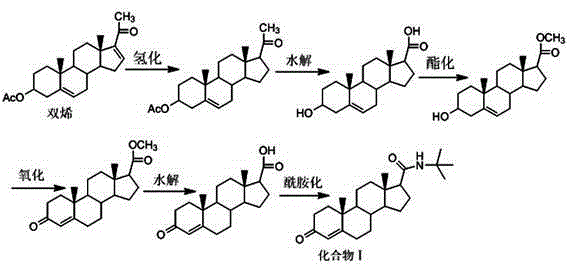
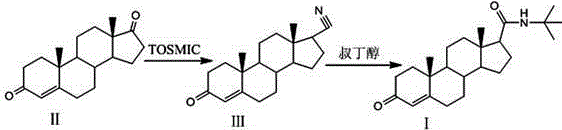
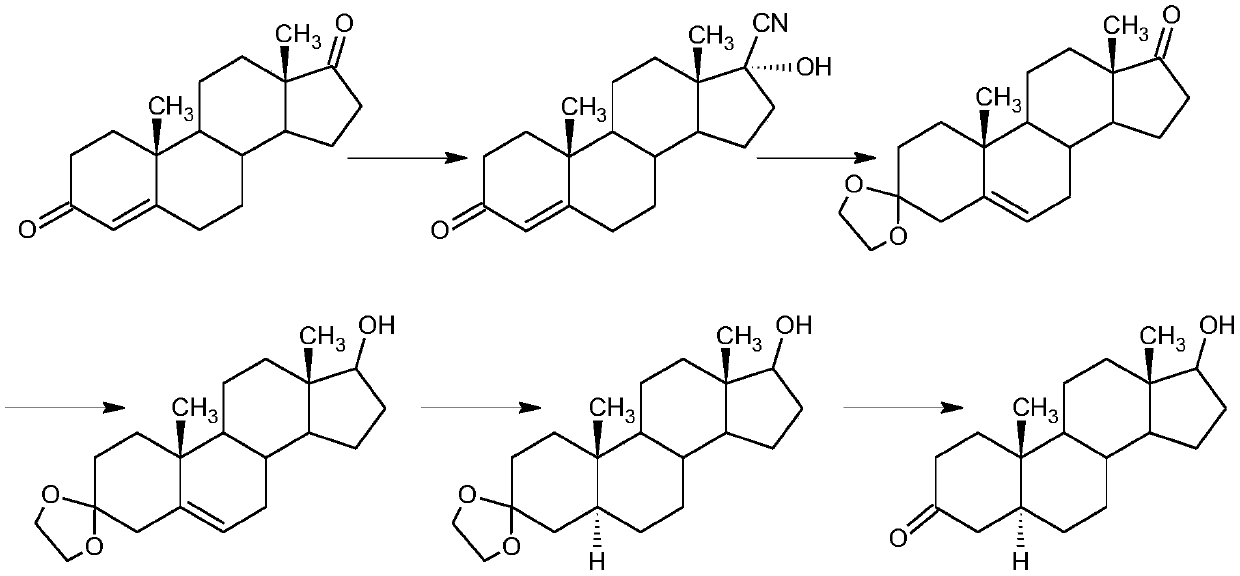
The invention relates to a preparation method for finasteride intermediate. According to the method, a product compound I, namely N-tert-butyl-3-oxo-4-olefinic-17 beta-finasteride formamide intermediate, is prepared by carrying out two steps of cyaniding and amidation on a compound II, namely androstenedione. A reaction scheme is shown in the specification. 4-androstenedione is used as a raw material, the resource is richer, and the cost is lower; the synthesis time is short, and the obtained finasteride intermediate is high in purity yield, and the HPLC is greater than or equal to 98%; and the yield is greater than or equal to 110%.
Owner:湖北竹溪人福药业有限责任公司
Method of synthesizing testosterone through 4-androstenedione one-step method
The invention provides a method of synthesizing testosterone through the 4-androstenedione one-step method. The method is characterized by comprising the following steps: Step 1, dissolving androstenedione in a mixed solvent system which is 4-14 times heavier than the androstenedione in weight, and cooling the mixture below minus 10 DEG C; Step 2, dissolving potassium borohydride in purified water which is 7-14 times heavier than potassium borohydride in weight; Step 3, adding the solution prepared in the Step 2 to the feed liquid which is obtained in the Step 1, 4-14 times heavier than the androstenedione and is at minus 5 to minus 10 DEG C; Step 4, taking a reaction at a condition of minus 5 to minus 10 DEG C until androstenedione is completely reacted, adding glacial acetic acid which is 15-40% of the weight of the potassium borohydride to demage excessive potassium borohydride, reducing the pressure, recovering the solvent, adding water, filtering and obtaining a crude product; Step 5, recrystallizing the crude product with ethanol to obtain the finished testosterone product.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
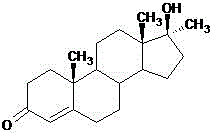
Preparation method of 5alpha-androstane-17-hydroxy-3-one
The invention relates to a preparation method of 5alpha-androstane-17-hydroxy-3-one. The method specifically comprises the following steps: 1, carrying out an etherification reaction on 4-androstenedione, triethyl orthoformate and anhydrous ethanol in the presence of an etherification catalyst to prepare a compound wet 3-ethoxy-3,5-androstadien-17-one; 2, sequentially adding a protective reagent and the wet 3-ethoxy-3,5-androstadien-17-one into a methanol-dichloromethane mixed solvent, and then carrying out catalytic hydrogenation reaction under the action of a palladium-carbon catalyst to obtain a 3-ethoxy-3-androstene-17-one solution; and 3, adding a metal borohydride into the 3-ethoxy-3-androstene-17-one solution, carrying out a reduction reaction, adding an acid after the reduction reaction is finished, carry out a hydrolysis reaction, removing the solvent after the hydrolysis reaction is finished, and carrying out filtering, water washing, drying, and refining to obtain the 5alpha-androstane-17-hydroxy-3-one. The method has the advantages of short process route, easily controlled production process, environmental friendliness, low production cost, and suitableness for industrial large-scale production.
Owner:HUAZHONG PHARMA
Treatment method for methyltestosterone mother liquor
The invention discloses a treatment method for methyltestosterone mother liquor. The treatment method comprises the following steps that 1, an ethyl acetate evaporation solvent is added into the methyltestosterone mother liquor, then, a mixed solvent, acetone cyanohydrin and organic aliphatic amine are added for reacting, and an intermediate material is obtained; 2, methyl alcohol is added into the intermediate material obtained in the first step, after uniform stirring, alkaline water is added for reacting, and crude 4-androstenedione is obtained; crude 4-androstenedione is refined, and refined 4-androstenedione is obtained through concentration, freezing and filtering; filter liquor obtained through filtering in the refining process of refined 4-androstenedione is subjected to concentration, freezing, filtering and recrystallization, and methyltestosterone is obtained. By means of the method, emissions of hormone waste are reduced, and pollution to the environment is reduced; 4-androstenedione and methyltestosterone can be recycled, recycled 4-androstenedione can be utilized again for methyltestosterone production, resource waste is avoided, and the production cost is greatly reduced.
Owner:HUAZHONG PHARMA
Method for converting and purifying 4-androstenedione from mother solutions of allyl ester substances and ketal substances
The invention discloses a method for converting and purifying 4-androstenedione from mother solutions of allyl ester substances and ketal substances. According to the method, 4-androstenedione is prepared from an allyl ester mother solution and a ketal substance mother solution which are prepared during the preparation of dehydroepiandrosterone, so that the repeated use of waste is realized, and the method is environmentally friendly and economic; the reaction route is greatly shortened, so that the generation of intermediate side reaction products is prevented, and the product purify and yield are high; and furthermore, the consumption of the reaction raw materials is relatively low, so that the cost is saved, and the requirement of green production idea is met.
Owner:湖北省丹江口开泰激素有限责任公司
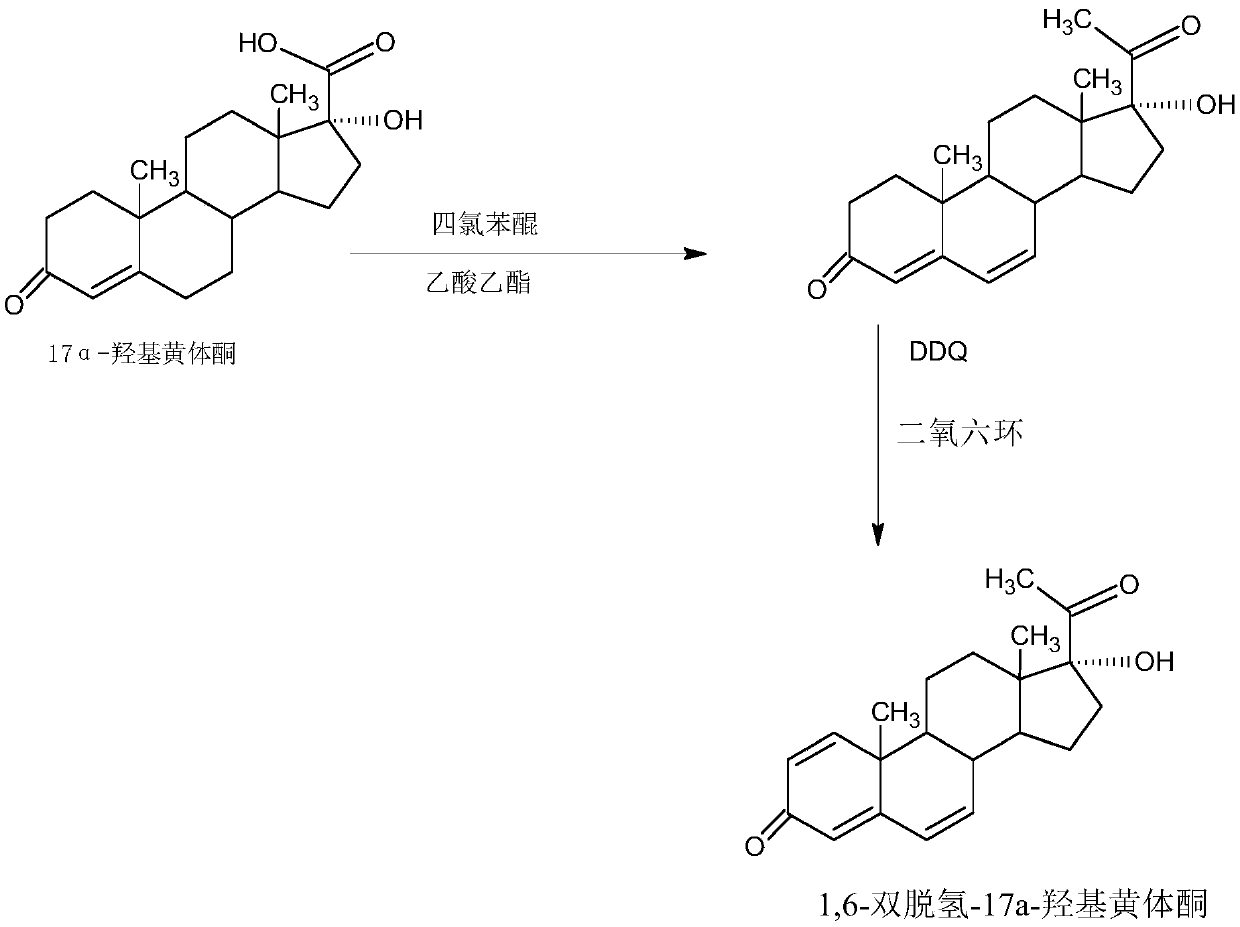
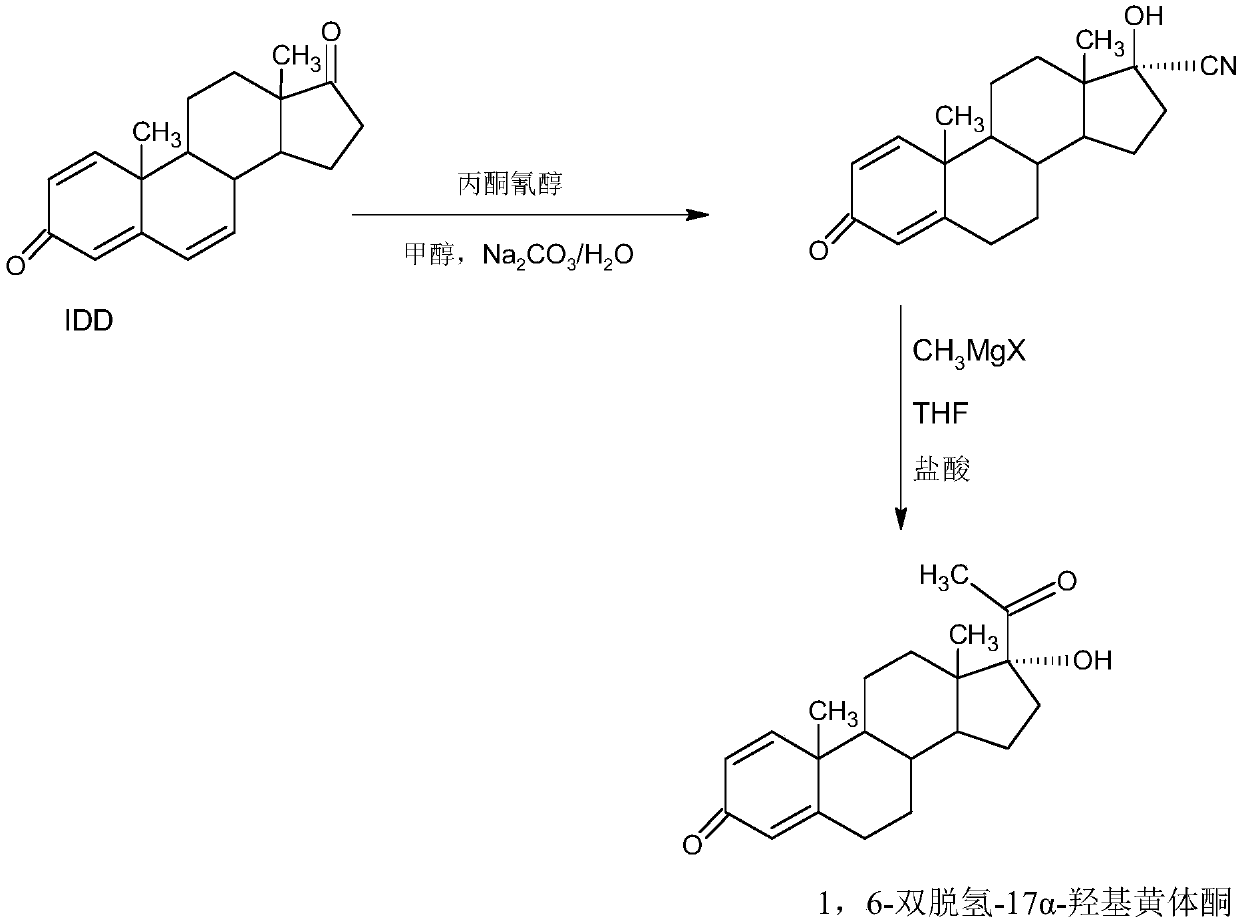
Preparation method of 1,6-didehydrogenation-17a-hydroxyl progesterone product
The invention provides a preparation method of a 1,6-didehydrogenation-17a-hydroxyl progesterone product. The preparation method includes the steps that 1,4-androstenedione (IDD) is adopted as a raw material, firstly, IDD molecules and acetone cyanohydrin react in a first organic solvent under the catalyzation of alkali, so that a hydroxyl-cyanogen product is obtained; then the hydroxyl-cyanogen product is prepared into 1,6-didehydrogenation-17a-hydroxyl progesterone under the existence of methyl magnesium halide, a second organic solvent and acid; then 1,6-didehydrogenation-17a-hydroxyl progesterone is subjected to heating reflux and decoloration in methylbenzene, acetone or lower alcohol of C4 or below and recrystallized, so that the1,6-didehydrogenation-17a-hydroxyl progesterone productis obtained. With the IDD being the raw material, compared with the traditional production method that dioscin is used as a raw material, the preparation method has the advantages of being wide in source of the raw material, making the technology economic and environmentally friendly and lowering production costs substantially. In the preparation method, expensive and toxic DDQ and a chloranil dehydrogenating agent do not need to be used; the solvent used in the technology can be recycled and applied mechanically, economy and environmental protection are realized, and industrialized production is quite easy.
Owner:HUNAN KEREY BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com