Preparation method for dehydroepiandrosterone, and enzyme for preparation thereof
A technology of dehydroepiandrosterone and androstenedione, which is applied in the field of biomedicine, can solve the problems of complicated purification operation, great environmental damage, low efficiency and the like, and achieves the effects of simple process, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The embodiment of the present invention provides a method for preparing ketoreductase and glucose dehydrogenase, the ketoreductase and glucose dehydrogenase are the enzymes used in the preparation method of dehydroepiandrosterone, the specific experiment Steps include:
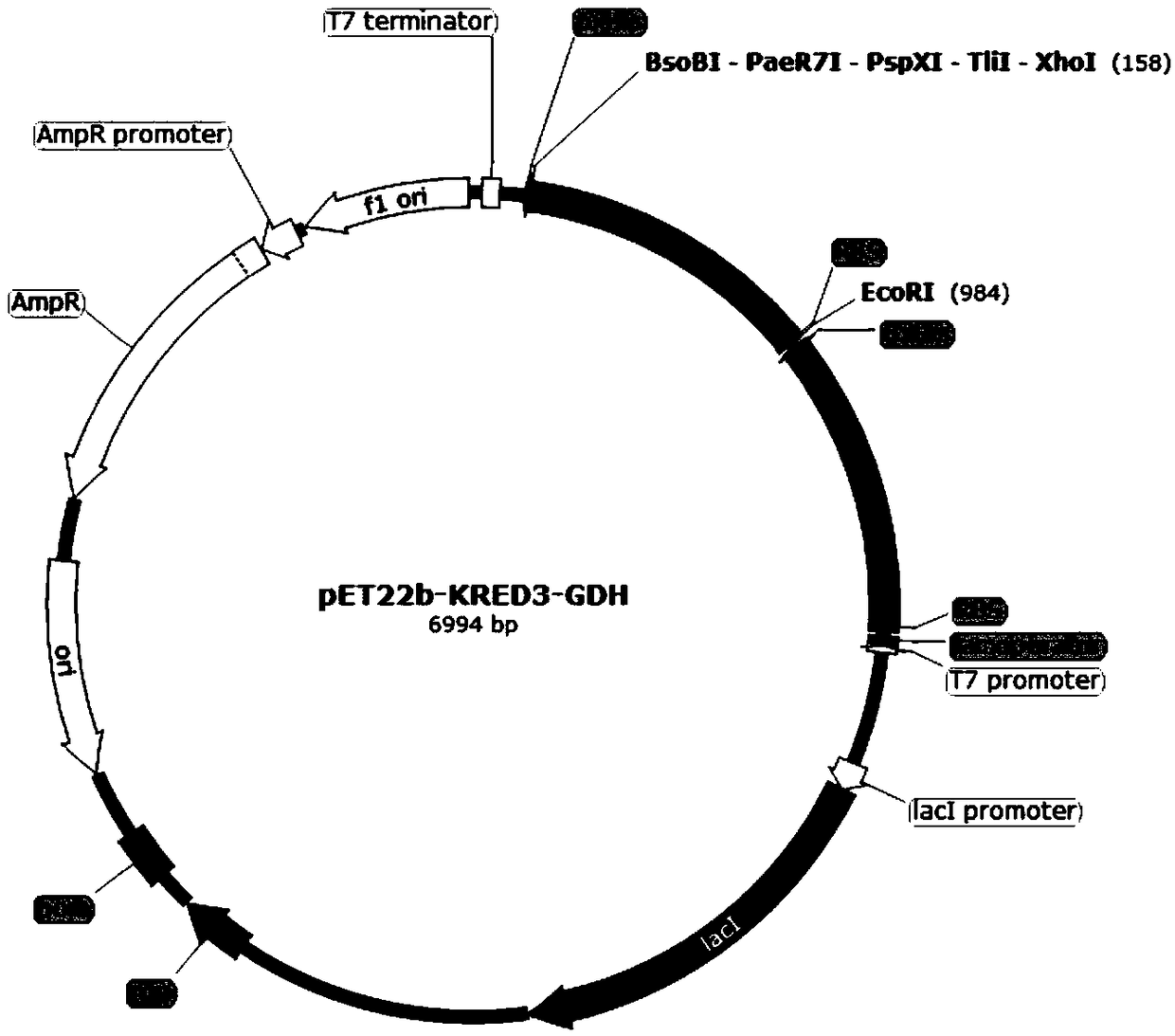
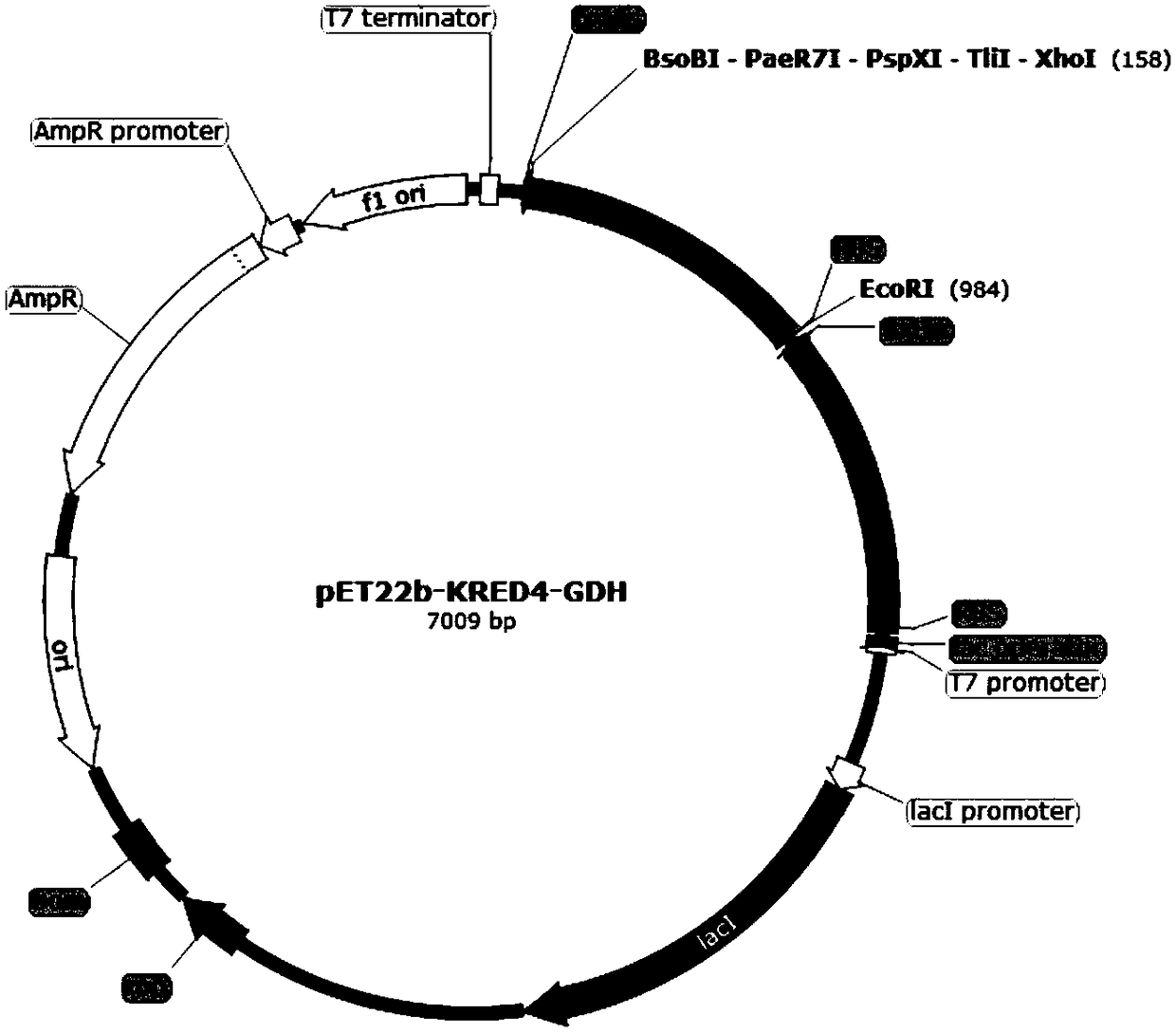
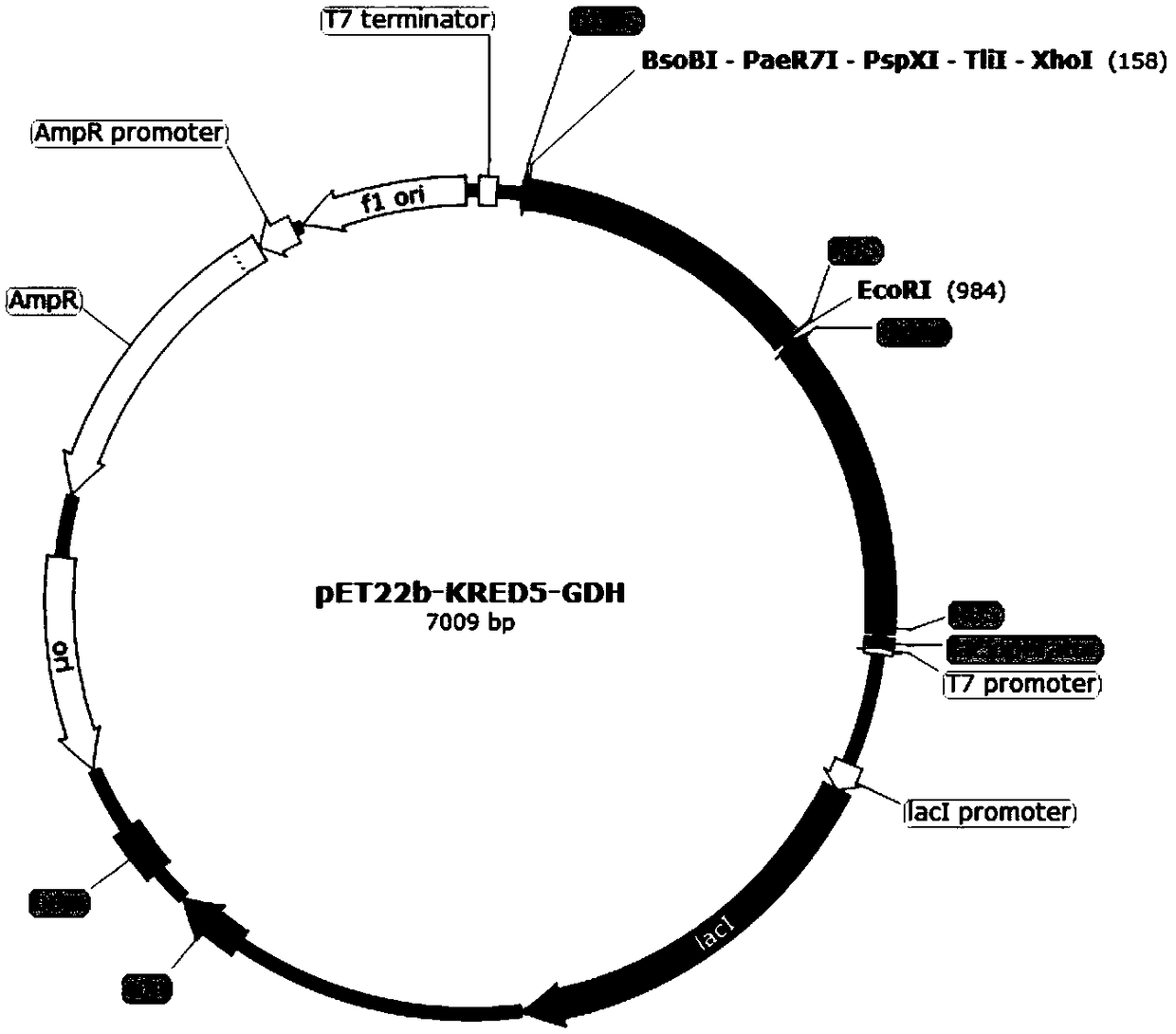
[0069] (1) Construction of pET22b-KRED3 / 4 / 5-GDH recombinant plasmid
[0070] Upstream primers and downstream primers are provided, and the gene coding sequences of ketoreductase KRED3, ketoreductase KRED4, ketoreductase KRED5 and glucose dehydrogenase are obtained by PCR amplification experiment. Wherein, the gene coding sequence of the ketoreductase KRED3 is shown in SEQ ID NO:1, the gene coding sequence of the ketoreductase KRED4 is shown in SEQ ID NO:2, and the gene coding sequence of the ketoreductase KRED5 As shown in SEQ ID NO:3, the gene coding sequence of the glucose dehydrogenase is shown in SEQ ID NO:4. The base sequence of the upstream primer corresponding to the ketoreductase KRED3 is show...
Embodiment 1
[0078] A preparation method for dehydroepiandrosterone, comprising the following steps:
[0079](1) Under the protection of nitrogen, add 82.5g of potassium tert-butoxide to 388mL of tert-butanol, then raise the temperature to 60°C, stir for 0.5h until the potassium tert-butoxide is completely dissolved, cool down to 30°C, and add 100g of 4-AD was stirred continuously for 0.5h, then cooled to room temperature to obtain a mixture. Under nitrogen protection, 6.0 g of sodium ascorbate was added to 205.3 g of 26.8% acetic acid aqueous solution to obtain an acetic acid solution containing sodium ascorbate. Under the protection of nitrogen, at 30°C, slowly drop the mixture into the acetic acid solution containing sodium ascorbate within 30 minutes, maintain the temperature at 30°C and continue stirring for 0.5h, and cool at room temperature to obtain a reaction containing 5-AD liquid.
[0080] (2) Add 500mL ethyl acetate to the 5-AD reaction solution, stir evenly, add 400mL 1.8g / L...
Embodiment 2
[0083] A preparation method for dehydroepiandrosterone, comprising the following steps:
[0084] (1) Under the protection of nitrogen, add 80.0g of potassium tert-butoxide to 388mL of tert-butanol, then raise the temperature to 55°C, stir for 0.8h until the potassium tert-butoxide is completely dissolved, cool down to 30°C, and add 100g of 4-AD was stirred continuously for 0.6h, then cooled to room temperature to obtain a mixture. Under nitrogen protection, 6.0 g of sodium ascorbate was added to 200.0 g of 26.8% acetic acid aqueous solution to obtain an acetic acid solution containing sodium ascorbate. Under the protection of nitrogen, at 30°C, slowly drop the mixture into the acetic acid solution containing sodium ascorbate within 30 minutes, maintain the temperature at 30°C and continue stirring for 0.5h, and cool at room temperature to obtain a reaction containing 5-AD liquid.
[0085] (2) Add 500mL ethyl acetate to the 5-AD reaction solution, stir evenly, add 400mL 1.5g / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com