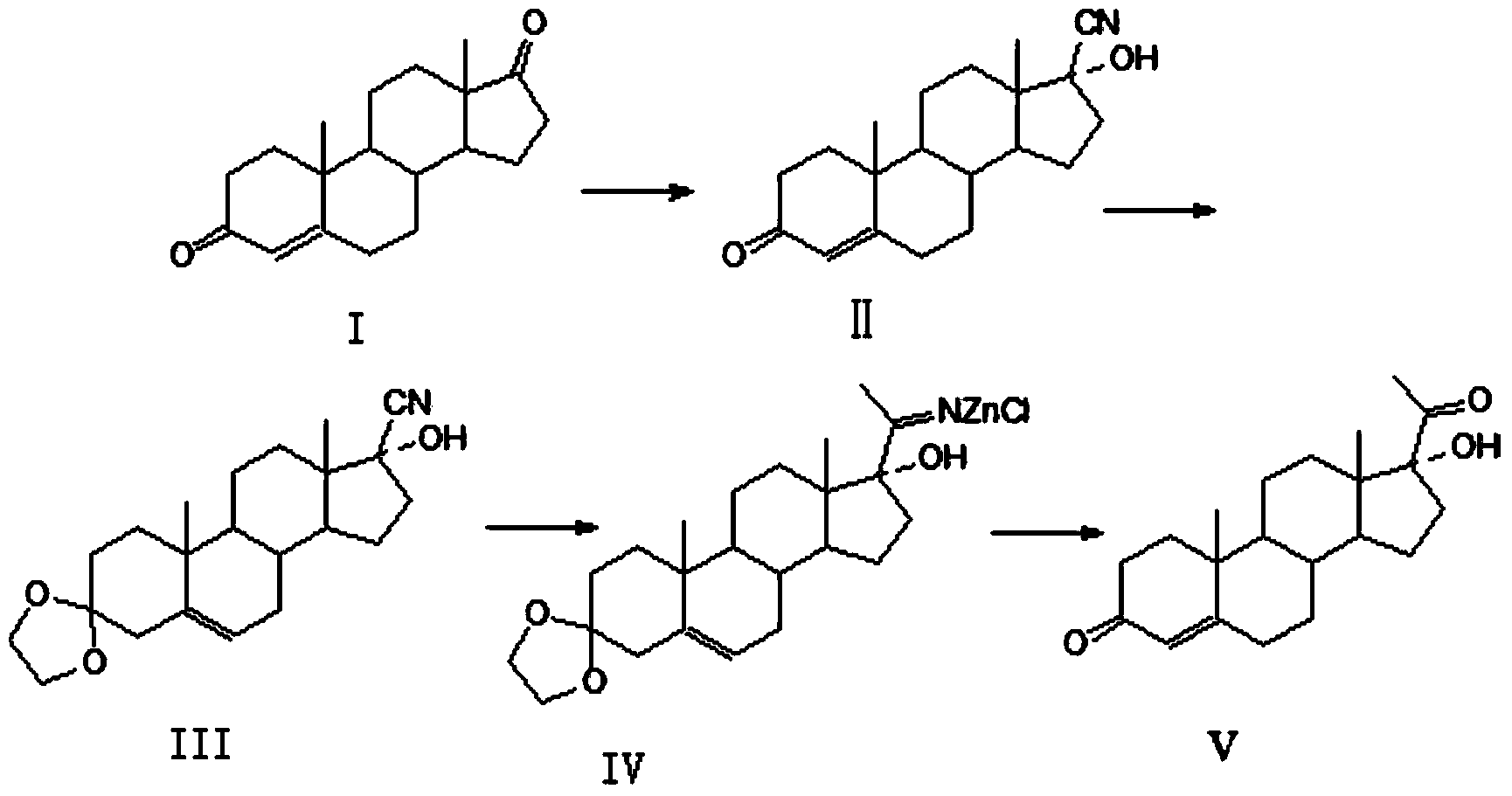
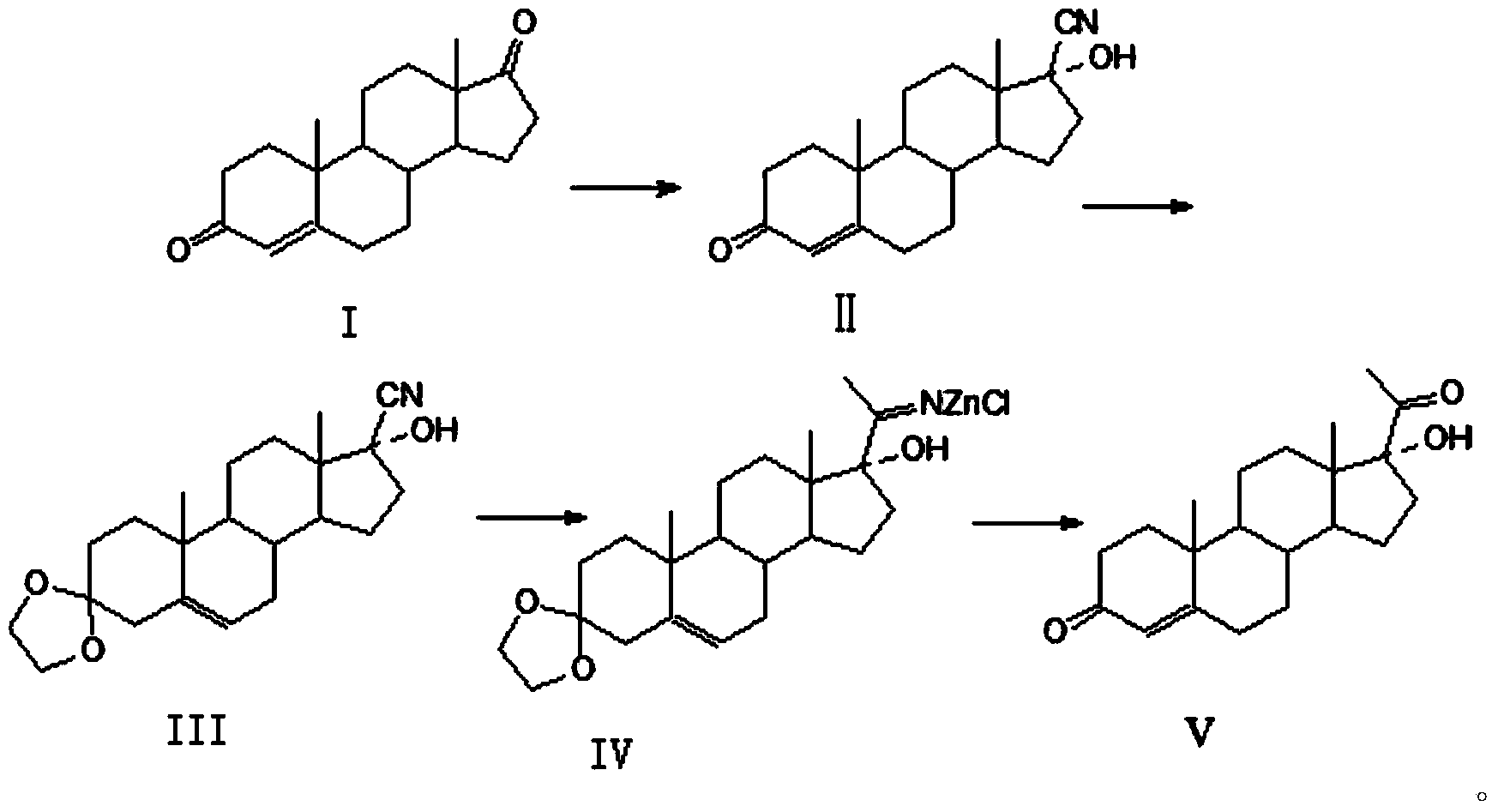
High-yield simple preparation method of 17alpha-hydroxy progesterone
A simple and simple technology for hydroxyprogesterone, which is applied in the field of medicine and chemical industry to achieve the effects of simplified process, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、17
[0031] The preparation of embodiment 1,17α-hydroxyprogesterone
[0032] 1. Add 50kg of methanol, 50kg of 4‐androstenedione, 100kg of acetone hydroalcohol into the reaction kettle, add 100kg of potassium hydroxide solution with a mass fraction of 0.1%, stir, control the temperature in the kettle to 50°C, and keep it warm at 50°C After the reaction was completed, the temperature was lowered to 0°C, filtered, washed with water, and dried to obtain 52.5kg of yellow solid 17-αhydroxyl-17-βcyanoandrost-4-en-3-one (II). 95.9%, HPLC content 98.5%.
[0033] Get the obtained 17-alpha hydroxy-17-beta cyanoandrost-4-en-3-one (II) 50kg and add in the reactor, then add ethylene glycol 150kg, triethyl orthoformate 100kg, p-toluenesulfonic acid Monohydrate 1.0kg, react at a temperature of 10-15°C for 24 hours, and the reaction is complete. Add sodium hydroxide solution, adjust pH=7-8, press filter, wash with water, and dry to obtain 55 kg of off-white solid ketal product (Ⅲ), the yield is 9...
Embodiment 2
[0036] As described in Example 1, the difference is that the initiator in step 2 is zinc chloride to obtain 45kg of 17α-hydroxyprogesterone, with a yield of 90.9%, the product appearance is white or off-white crystalline powder, the HPLC purity is 99.5%, and the melting point is: 219~220℃.
Embodiment 3
[0038] As described in Example 1, the difference is that the reflux time of step 2 is 11 hours, 45 kg of 17α-hydroxyprogesterone is obtained, the yield is 97.4%, the product appearance is white or off-white crystalline powder, the HPLC purity is 99.6%, and the melting point: 219.1-219.5 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com