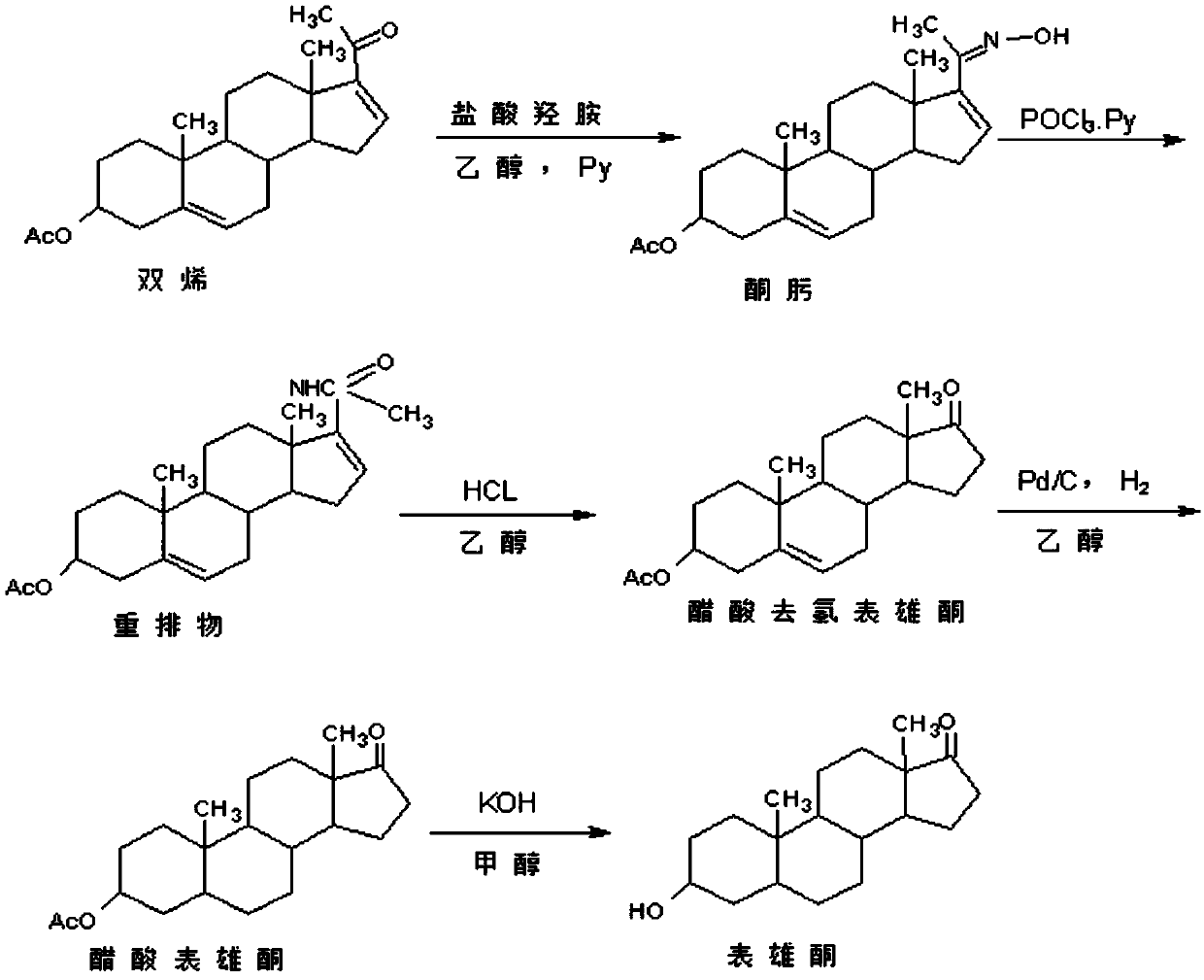
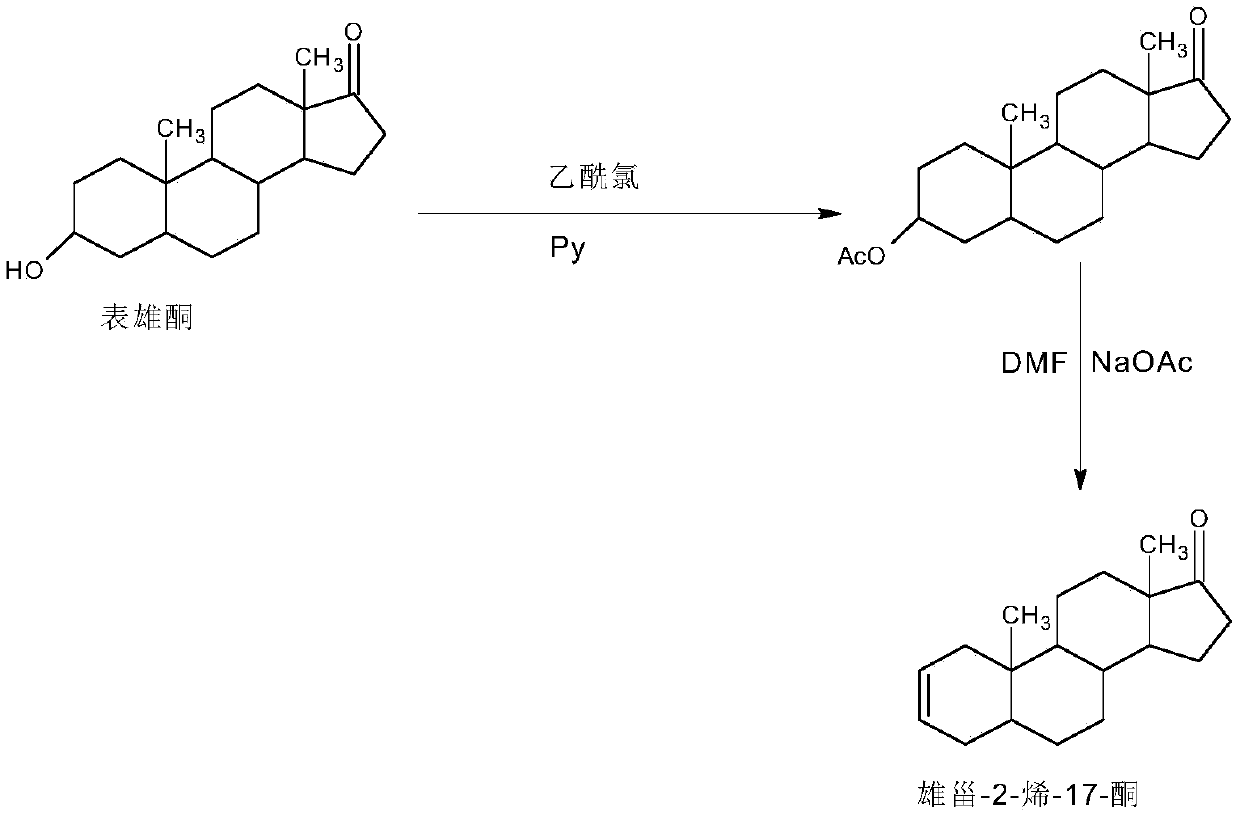
Preparation method of androst-2-en-17-one
A technology of androstenedione and androstene is applied in the field of preparation of steroid hormone drug intermediates, can solve the problems of large side reactions, large amount of by-products, low synthesis yield and the like in the elimination reaction, and achieves good product quality and high reaction efficiency. Selective and source-rich effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A, preparation of solid-phase acid catalyst:
[0030] In a 1000ml three-neck flask, add 100g of solid silica gel, 400ml of pure water, 200ml of pure water and 5g of p-toluenesulfonic acid, and stir the adsorption reaction at 30-35°C for 3-4 hours. After the reaction, evaporate under reduced pressure. Remove water to nearly dry, take out and dry to obtain 99.8g of solid phase acid catalyst, moisture content 2.6%, weight yield 99.8%;
[0031] B, 4-androstenedione to prepare epiandrosterone:
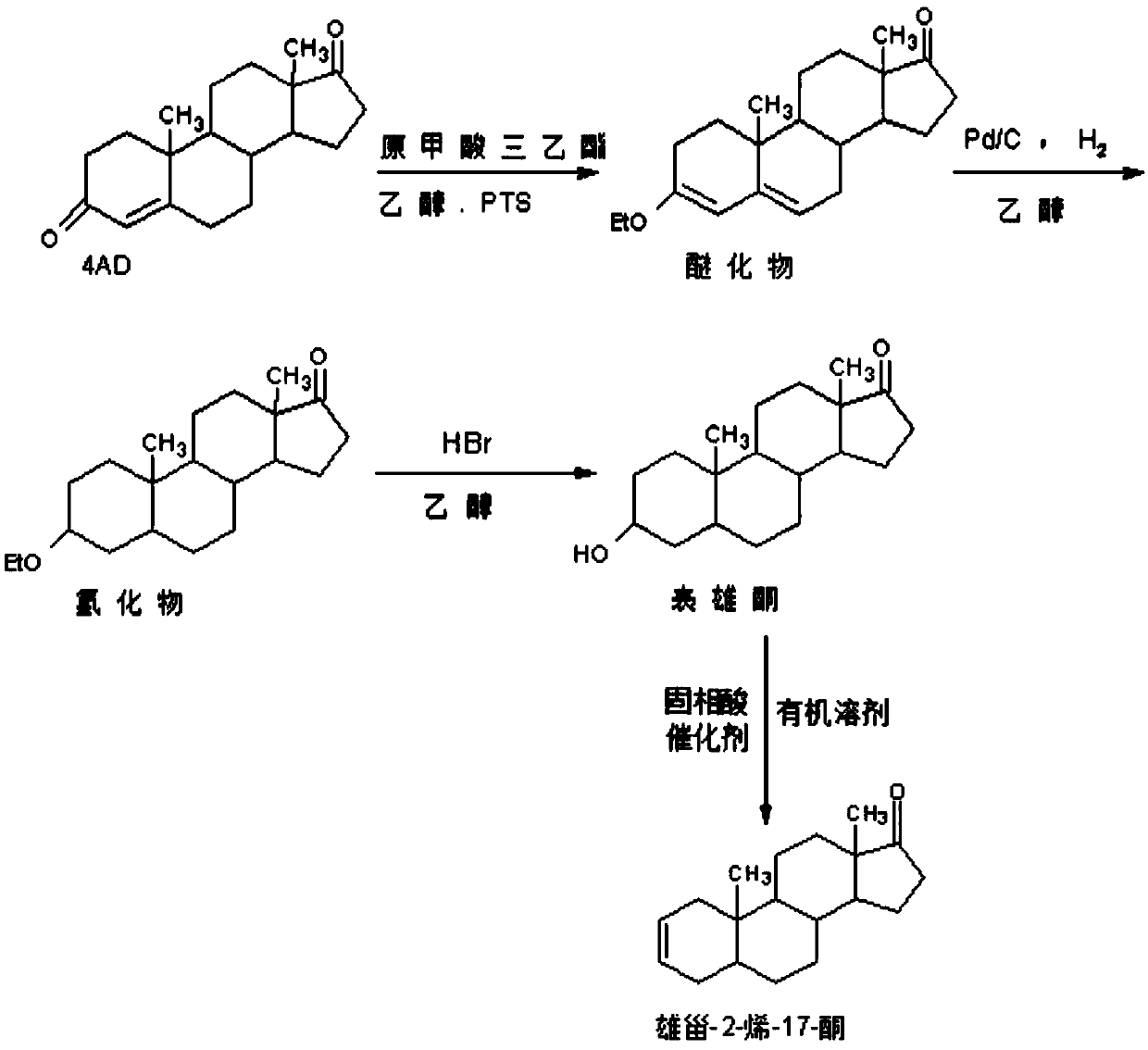
[0032] Such as image 3 As shown, using 4-androstenedione as a raw material, epiandrosterone was synthesized through a three-step reaction of etherification protection reaction, palladium carbon-catalyzed hydrogenation reduction reaction, and acid-catalyzed hydrolysis deprotection reaction. Specifically, 4-androstenedione is firstly used as a raw material to react with triethyl orthoformate to generate an etherified product protected by etherification at the 3-position, and the eth...
Embodiment 2
[0036] A, preparation of solid-phase acid catalyst:
[0037] In a 1000ml three-necked flask, add 100g of solid alumina, 400ml of pure water, 200ml of pure water and 5g of sulfuric acid, and stir the adsorption reaction at 30-35°C for 3-4 hours. After the reaction, evaporate the water under reduced pressure. To nearly dry, take out and dry to obtain 99.6g of solid-phase alkali catalyst, moisture content 2.8%, weight yield 99.6%;
[0038] B, 4-androstenedione to prepare epiandrosterone:
[0039] Such as image 3 As shown, using 4-androstenedione as a raw material, epiandrosterone was synthesized through a three-step reaction of etherification protection reaction, palladium carbon-catalyzed hydrogenation reduction reaction, and acid-catalyzed hydrolysis deprotection reaction. Specifically, 4-androstenedione is firstly used as a raw material to react with triethyl orthoformate to generate an etherified product protected by etherification at the 3-position, and the etherified pro...
Embodiment 3
[0043] A, preparation of solid-phase acid catalyst:
[0044] In a 1000ml three-necked flask, add 100g of solid magnesium sulfate powder, 400ml of pure water, 200ml of pure water and 5g of nitric acid, and stir the adsorption reaction at 30-35°C for 3-4 hours. After the reaction, evaporate it under reduced pressure. Water to nearly dry, take out and dry to obtain 99.8g of solid-phase acid catalyst, moisture content 2.1%, weight yield 99.8%;
[0045] B, 4-androstenedione to prepare epiandrosterone:
[0046] Such as image 3 As shown, using 4-androstenedione as a raw material, epiandrosterone was synthesized through a three-step reaction of etherification protection reaction, palladium carbon-catalyzed hydrogenation reduction reaction, and acid-catalyzed hydrolysis deprotection reaction. Specifically, 4-androstenedione is firstly used as a raw material to react with triethyl orthoformate to generate an etherified product protected by etherification at the 3-position, and the et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com