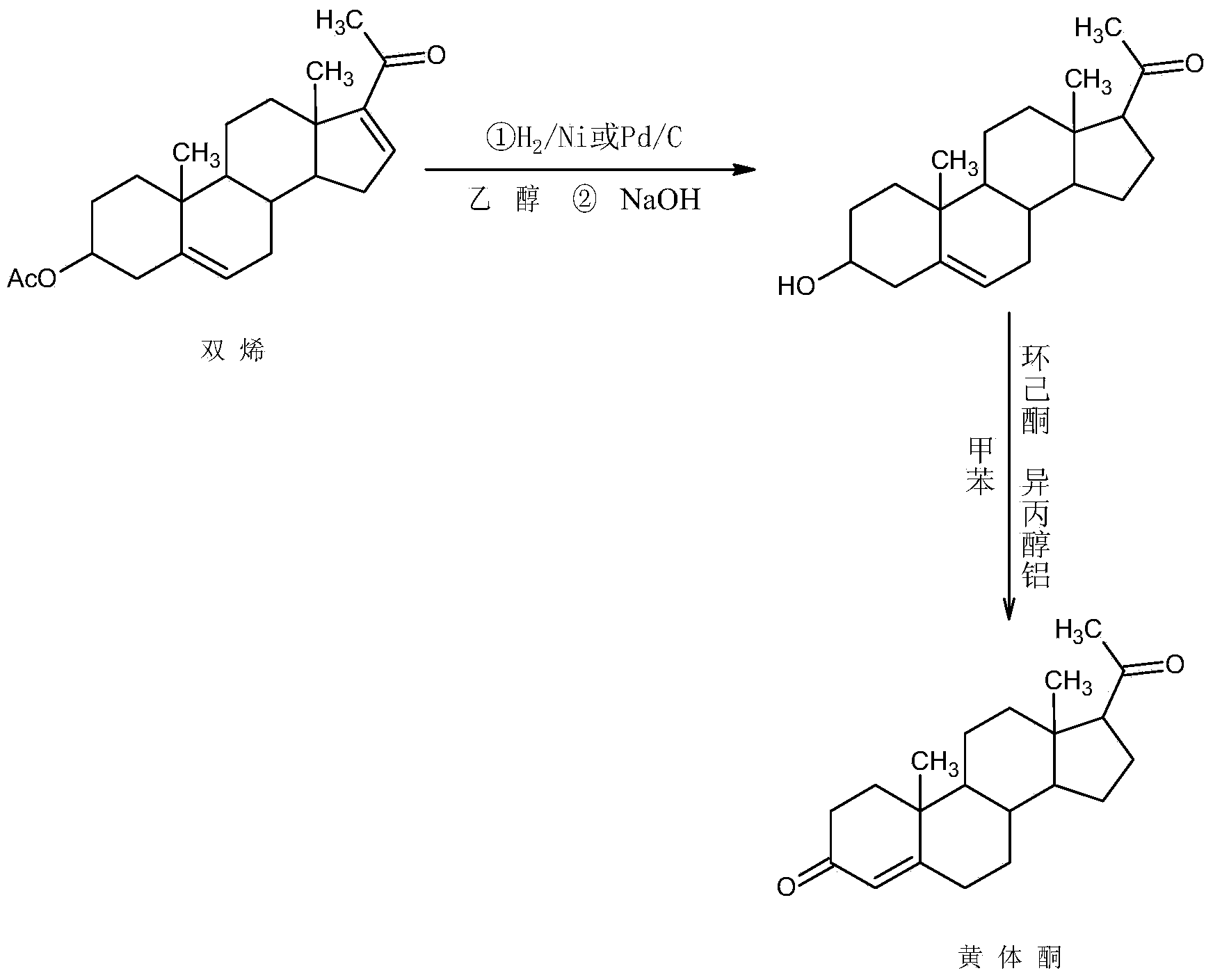
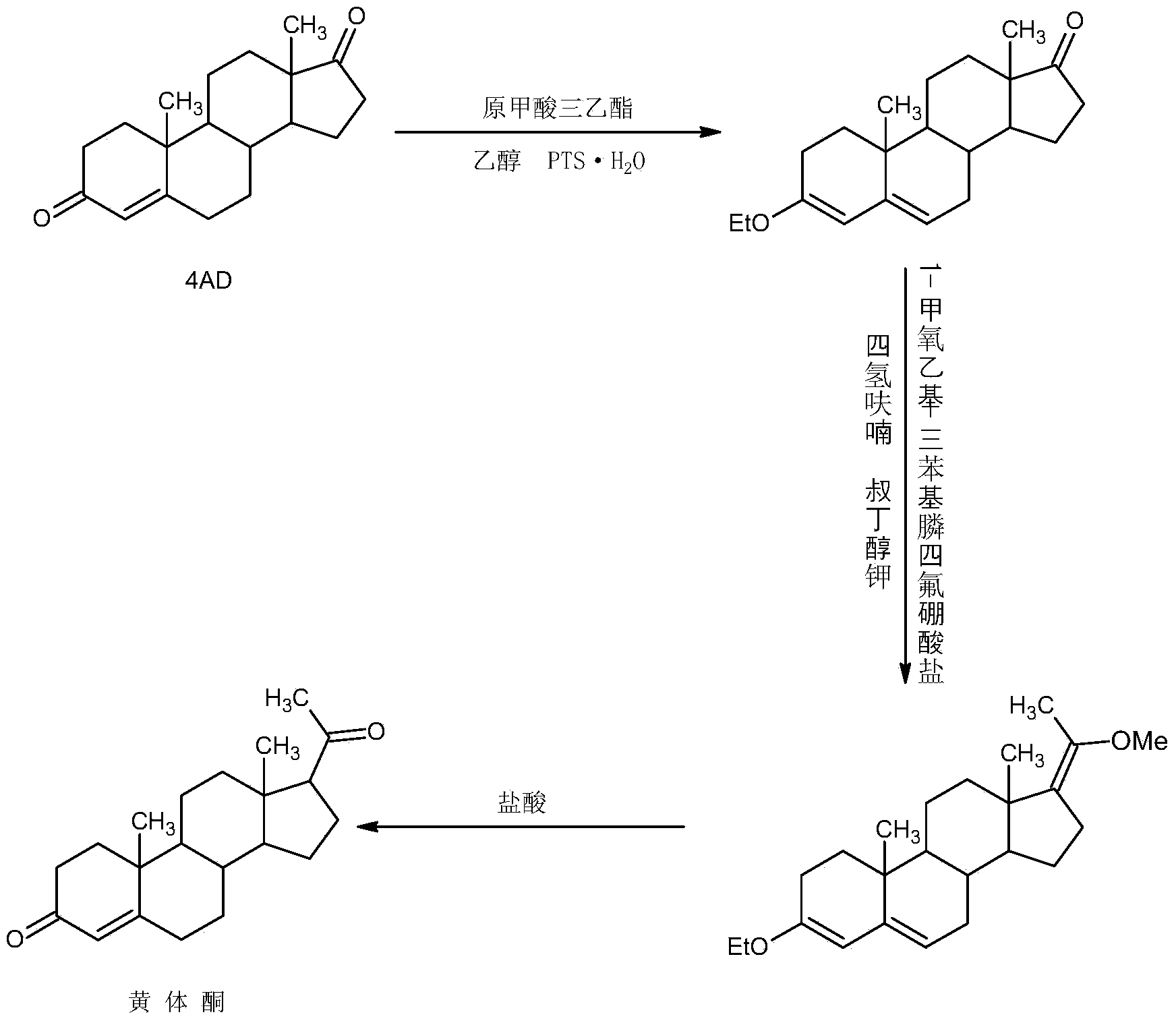
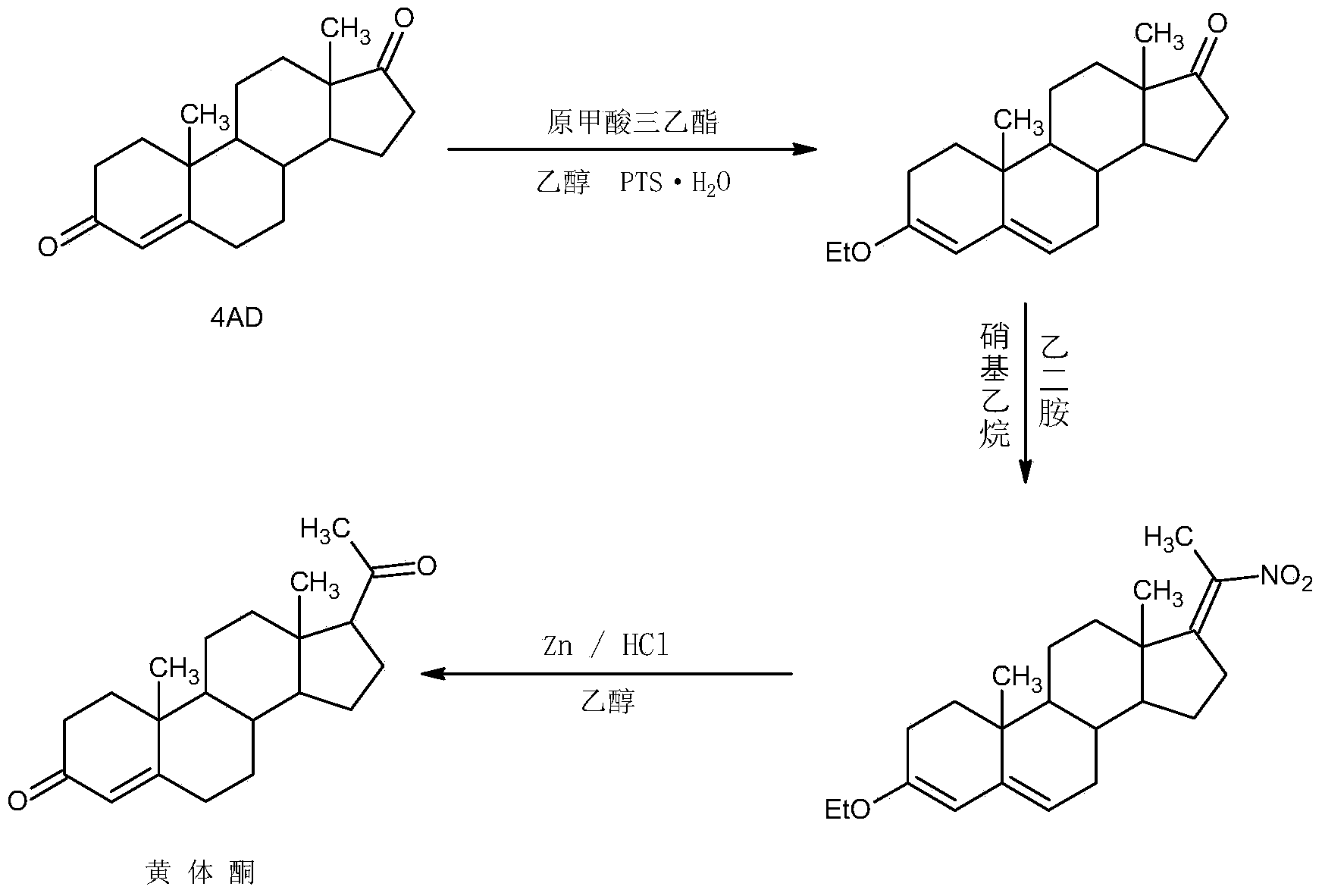
Preparation method for progestin
A technology of progesterone and androstenedione, which is applied in the field of preparation of the progesterone drug progesterone, can solve the problems of not obvious cost advantages, difficult purification, poor quality, etc., and achieve low cost, wide source of raw materials, and good quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A. Preparation of ether compounds
[0028] In a 1000ml three-necked flask, add 100g 4AD, 200ml ethanol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 20-25°C and stir for 12-16 hours. TLC detects the end point of the reaction. After the reaction, add 3ml Pyridine, stir for 20-25 minutes to neutralize the acid, then cool the system to -5-0°C, stir and crystallize for 2-3 hours, filter with suction, wash with a small amount of ethanol, combine the lotion and filtrate, recover the solvent and use the crude product mechanically; filter cake Drying below 70°C gave 101.6g of ether compound with HPLC content of 99.2% and weight yield of 101.6%.
[0029] B. Preparation of nitro compounds
[0030]In a 1000ml three-necked flask, add 100g of ether compound, 400ml of nitroethane, and 5ml of ethylenediamine, keep warm at 85-90°C and stir for 6-8 hours. TLC detects the end point of the reaction. After the reaction, concentrate under reduced pressure and recover...
Embodiment 2
[0034] A. Preparation of ether compounds
[0035] In a 1000ml three-neck flask, add 100g4AD, 600ml dichloromethane, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 20-25°C and stir for 12-16 hours. TLC detects the end point of the reaction. After the reaction, Add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, concentrate under reduced pressure, recover dichloromethane, lower the temperature, add 100ml of ethanol, then cool the system to -5-0°C, stir and crystallize for 2-3 hours, suction filter, a small amount Wash with ethanol, combine the lotion and filtrate, recover the solvent and reuse the crude product; dry the filter cake below 70° C. to obtain 100.2 g of ether compound, the HPLC content is 99.4%, and the weight yield is 100.2%.
[0036] B. Preparation of nitro compounds
[0037] In a 1000ml three-necked flask, add 100g of ether compound, 60ml of nitroethane, 600ml of toluene, and 5ml of ethylenediamine, keep warm at 85-90°C and st...
Embodiment 3
[0041] A. Preparation of ether compounds
[0042] In a 1000ml three-neck flask, add 100g 4AD, 200ml ethanol, 80ml triethyl orthoformate, add 2g of HCl gas, seal it, keep it warm at 2025°C and stir for 12-16 hours. TLC detects the reaction end point. After the reaction, add 3ml Pyridine, stir for 20-25 minutes to neutralize the acid, then cool the system to -5-0°C, stir and crystallize for 2-3 hours, filter with suction, wash with a small amount of ethanol, combine the lotion and filtrate, recover the solvent and apply the crude product; filter cake After drying below 70°C, 103.2 g of the ether compound was obtained, the HPLC content was 99.1%, and the weight yield was 103.2%.
[0043] B. Preparation of nitro compounds
[0044] In a 1000ml three-neck flask, add 100g of ether compound, 400ml of nitroethane, and 5ml of N,N-tetramethylethylenediamine, keep warm at 85-90°C and stir for 6-8 hours. TLC detects the end point of the reaction. , concentrated under reduced pressure, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com