Technology for refining 4-androstenedione from dehydroepiandrosterone mother solution
A technology of dehydroepiandrosterone and androstenedione, which is applied in the field of processing dehydroepiandrosterone mother liquor, can solve the problems of low yield of 4-androstenedione, and achieve environmental protection and reduce solid The effect of waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
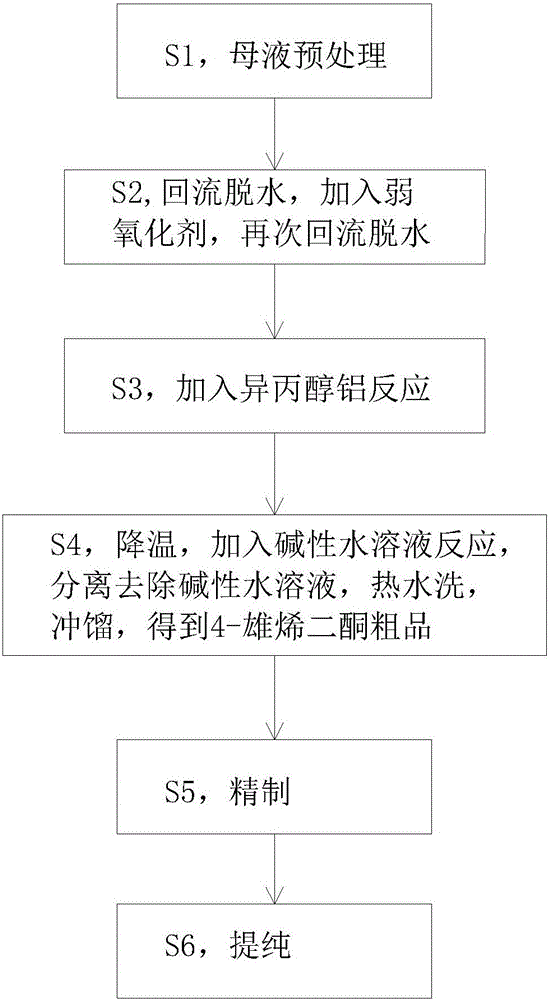
[0037] S1, take 100kg of dehydroepiandrosterone mother liquor obtained in the process of synthesizing dehydroepiandrosterone with 4-androstenedione, add 1000kg of toluene, heat and dissolve it at 50°C, and use 20kg of methanol + 180kg of water + 20kg of hydrochloric acid to form Adjust the pH value of the solution to 2, stir at 55°C for half an hour, separate the water in the lower layer, and wash the upper layer with hot water until the pH is 7;
[0038] S2, heating the upper layer liquid obtained in step S1 to 112°C, reflux for dehydration, then lower the temperature to 80°C, add cyclohexanone, and reflux for dehydration until the effluent is clear;
[0039] S3, after the dehydration is completed, cool down to 110°C, add aluminum isopropoxide, slowly raise the temperature to 114°C, react for 5 hours, take samples for TLC spot detection until the reaction is complete;
[0040] S4, after the reaction is completed, lower the temperature to 80°C, add NaOH aqueous solution, and r...
Embodiment 2
[0045] S1, take 100kg of dehydroepiandrosterone mother liquor obtained in the process of synthesizing dehydroepiandrosterone with 4-androstenedione, add 1000kg of ethyl acetate, heat and dissolve at 50°C, use 30kg of ethanol + 280kg of water + 30kg The acid solution composed of hydrochloric acid adjusts the pH value of the solution to 2.5, stirs at 52°C for half an hour, separates the water in the lower layer, and washes the upper layer with hot water until the pH is 6.5;
[0046] S2, heating the upper layer liquid obtained in step S1 to 111°C, reflux for dehydration, then lower the temperature to 85°C, add cyclohexanone, and reflux for dehydration until the effluent is clear;
[0047] S3, after the dehydration is completed, cool down to 105°C, add aluminum isopropoxide, slowly raise the temperature to 112°C, react for 6 hours, take samples for TLC spot detection until the reaction is complete;
[0048] S4, after the reaction is completed, lower the temperature to 85°C, add Na...
Embodiment 3
[0053] S1, take 100kg of dehydroepiandrosterone mother liquor obtained in the process of synthesizing dehydroepiandrosterone with 4-androstenedione, add 1000kg of ethyl acetate, heat and dissolve at 55°C, use 50kg of ethanol + 480kg of water + 50kg The acid solution composed of hydrochloric acid adjusts the pH value of the solution to 3, stirs at 55°C for half an hour, separates the water in the lower layer, and washes the upper layer with hot water until the pH is 7;
[0054] S2, heating the supernatant liquid obtained in step S1 to 112°C, reflux for dehydration, then lower the temperature to 90°C, add calcium hypochlorite, and reflux for dehydration until the effluent is clear;
[0055] S3, after the dehydration is completed, cool down to 110°C, add aluminum isopropoxide, slowly raise the temperature to 114°C, react for 8 hours, take samples for TLC spot detection until the reaction is complete;
[0056] S4, after the reaction is completed, lower the temperature to 90°C, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com