Method for preparing dehydroepiandrosterone through chemical-enzyme method
A technology for the preparation of dehydroepiandrosterone and enzymatic method, which is applied in the field of chemical-enzymatic preparation of dehydroepiandrosterone, can solve the problems of difficult removal of impurities, high amount of organic solvent, high amount of potassium tert-butoxide, etc., and achieve high practicality The effect of high value, high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
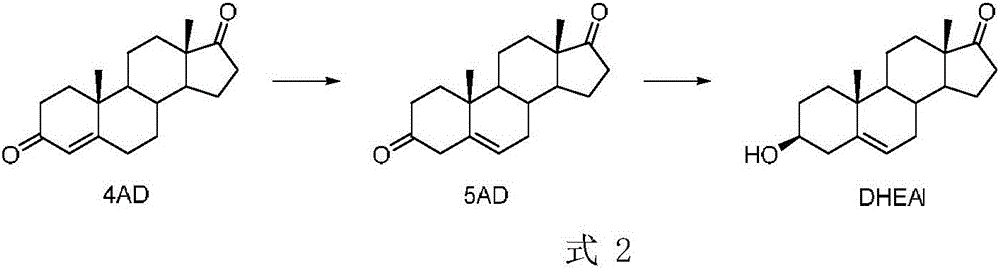
[0023] Example 1 Preparation of 5-AD
[0024] At 25° C., nitrogen was bubbled into tert-butanol (80 mL) for 15 minutes, potassium tert-butoxide (6.0 g, 3.0 eq) was added under aeration conditions, and the reactor was sealed, replaced with nitrogen, and stirred for half an hour. Under the protection of nitrogen, the substrate (5.0g, 1.0eq) was added, and the reaction was carried out at 35°C for 5 hours. Take 3.5 g of sodium ascorbate, 5.7 g of acetic acid, and 200 mL of water, stir for 15 minutes, pour the above-mentioned reaction solution, and stir for half an hour under nitrogen protection. The conversion rate detected by HPLC is 96.5%. The mixed solution was filtered to obtain a white solid, washed with water, and spin-dried to obtain 4.8 g of a white solid with a purity of 95.5% by HPLC and a yield of 91.7%.
Embodiment 2
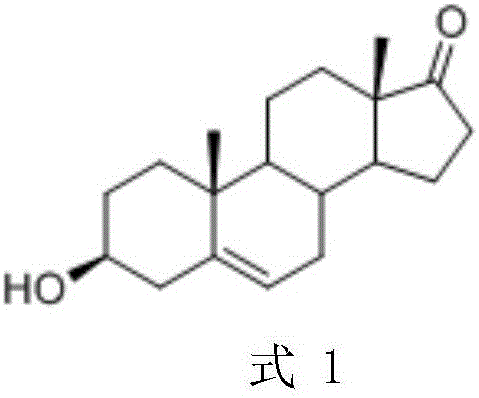
[0025] Example 2 Preparation of dehydroepiandrosterone
[0026] Dissolve the above 4.8g product in 25mL 2-methyltetrahydrofuran, add 25mL 100mM pH6.5 phosphate buffer solution to start stirring, add 5g glucose, 80mg magnesium chloride hexahydrate, 60mg ketoreductase (from sequence 26 in E. coli Obtained from medium expression), 30mg glucose dehydrogenase (from Suzhou Hanzyme Biotechnology Co., Ltd., numbered EW002), 20mg NAD, start the reaction at 25°C, and use 40% sodium hydroxide to control pH 6.5. After 6 hours, take a sample for detection. Conversion rate 99%, adjust pH with hydrochloric acid 99%, content> After 99% of the mother liquor is evaporated to dryness, 1.4 g of solid is obtained. After re-recycling, the multi-batch post-processing yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com