Synthesis method of spironolactone intermediate canrenone
A synthetic method and intermediate technology, applied in the field of chemical drug synthesis, can solve problems such as harsh reaction conditions, high equipment requirements, and low total yield, and achieve the effects of stable method, good selectivity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
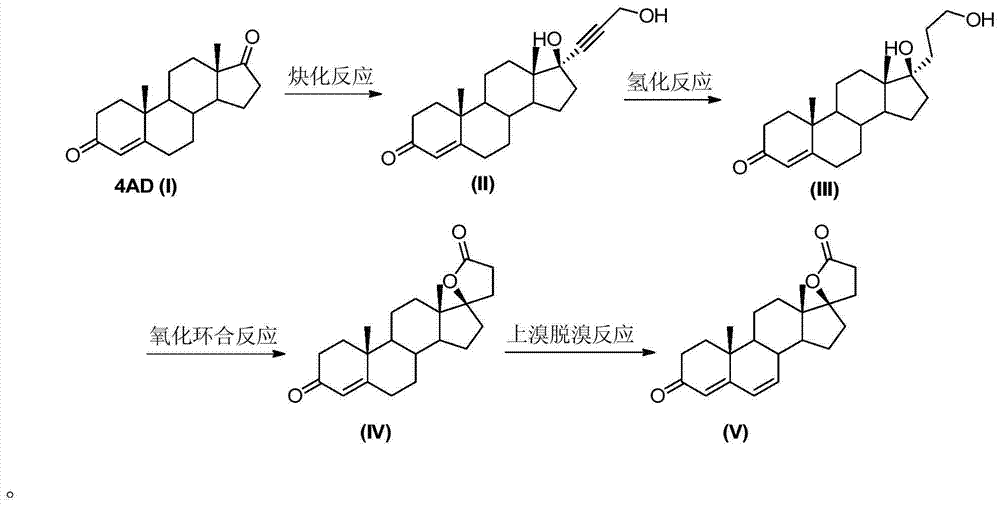
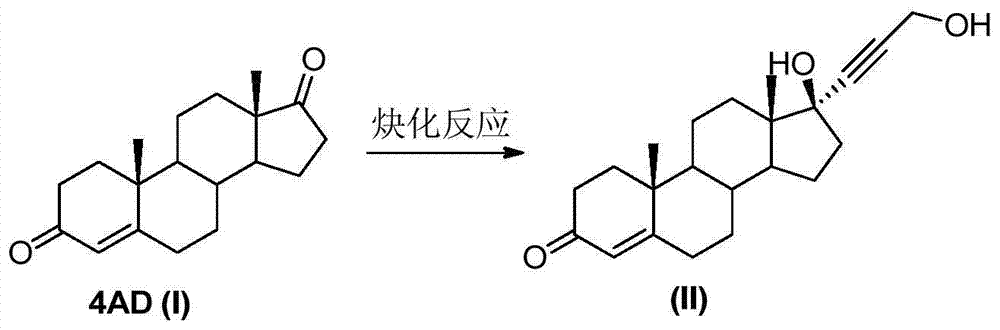
[0028] Embodiment 1: Alkynylation reaction
[0029] The reaction formula is as follows:
[0030]
[0031] Under the protection of nitrogen, add 28g of pre-crushed potassium hydroxide to the three-necked flask, add 200mL of solvent tetrahydrofuran and 10g of 4AD(I), stir evenly, control the temperature at 15-20°C, add 12mL of propynyl alcohol dropwise, and the dropwise addition time is about 0.5 hour, then the temperature was controlled at 15-20°C, and the reaction was carried out for about 6 hours, monitored by TLC until the reaction was complete. Add about 9 mL of hydrochloric acid aqueous solution (concentration about 2N) dropwise to the reaction solution, adjust the pH value to neutral, concentrate under reduced pressure, add 100 mL of water for water analysis, let stand for 0.5 hours, filter, and dry at 60°C for 24 hours to obtain 11.5 g of the compound (II), the mass yield is about 115%. Embodiment 2: Alkynylation reaction
Embodiment 2
[0032] Under the protection of nitrogen, add 25g of pre-crushed sodium hydroxide to the three-necked flask, add 200mL of solvent 2-methyltetrahydrofuran and 10g of 4AD(I), stir evenly, control the temperature at 15-20°C, add dropwise 12mL of propynyl alcohol, The dropwise addition time was about 0.5 hour, and then the temperature was controlled at 15-20°C, and the reaction was carried out for about 10 hours, monitored by TLC until the reaction was complete. Add about 12mL hydrochloric acid aqueous solution (concentration 2N) dropwise to the reaction solution, adjust the pH value to neutral, add 100mL water for water analysis after concentration under reduced pressure, let stand for 0.5 hour, filter, and dry at 60°C for 24 hours to obtain 10.8g compound ( II), the mass yield is about 108%.
Embodiment 3
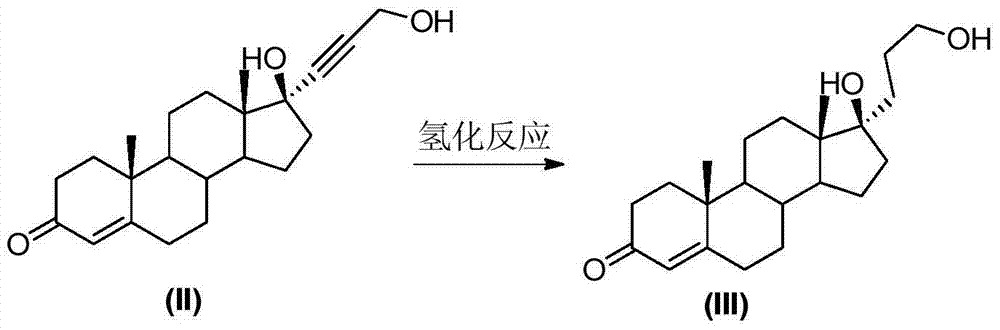
[0033] Embodiment 3: hydrogenation reaction
[0034] The reaction formula is as follows:
[0035]
[0036] Under the protection of nitrogen, add 10g of compound (II) and 200mL of ethanol to a three-neck flask, add 1g of activated carbon loaded with 5% palladium carbon, pass in hydrogen, and react at 20-30°C for about 5 hours. After the reaction is complete, stop the ventilation, filter, and the filtrate Concentrate under reduced pressure to nearly dryness, add 50 mL of water for water analysis, filter, and dry at 60°C to obtain 9.3 g of compound (III); the mass yield is about 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com