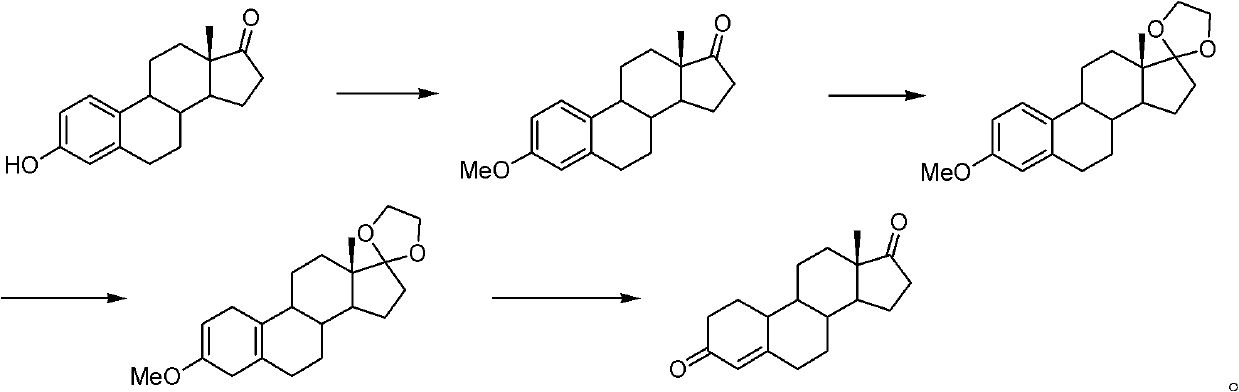
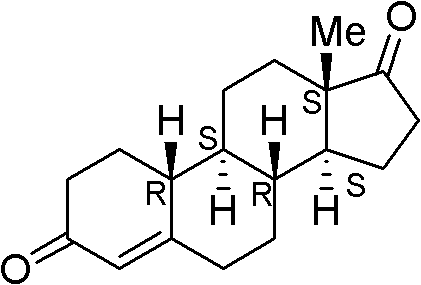
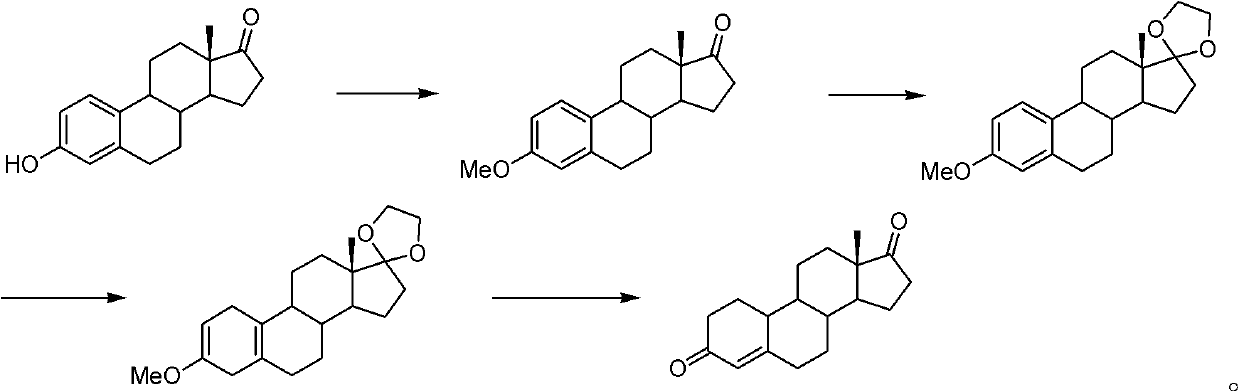
Method for preparing 19-demethyl-4-androstenedione
A technology of androstenedione and ketal, which is applied in the field of chemical preparation, can solve the problems of high price and high production cost, and achieve the effects of less by-products, increased yield and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] In the first step, add 10g of estrone and 15g of potassium carbonate into a 250ml reaction bottle, first add 100ml of solvent N,N-dimethylformamide at room temperature, then add 50ml of dimethyl carbonate, and react at 130°C for 16 hours After the reaction is completed, pour it into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60°C to obtain etherified compound with a yield of 104% and an HPLC content of 98%;
[0071] In the second step, add 20ml of ethylene glycol, 25ml of triethyl orthoformate, 0.2ml of boron trifluoride ether into a dry 250ml reaction bottle, stir at 25°C for 15 minutes, then add 10g of ether compound, 40ml of dichloromethane, 25 Ketal reaction at ℃ for 5 hours; TLC (thin layer chromatography) detected no raw material point, added triethylamine, stirred for 10 minutes; concentrated under reduced pressure until no distillate was evaporated, poured into ice water and stirred for one hour, filtered, washed w...
Embodiment 2
[0075] In the first step, add 10g of estrone and 20g of potassium carbonate to a 250ml reaction bottle, first add 100ml of solvent N,N-dimethylacetamide at room temperature, then add 50ml of dimethyl carbonate, and react at 100°C for 16 hours After the reaction is completed, pour into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60° C. to obtain the ether compound with a yield of 103% and an HPLC content of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com