Preparation method for hydroxylation of 11 alpha of important intermediate of steroidal hormone substance
A technology of steroid hormones and intermediates, which is applied in the field of microbial transformation of steroid drugs, can solve the problems of low conversion rate and unfriendly environment, and achieve the effects of high conversion efficiency, improved utilization rate, and shortened production cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
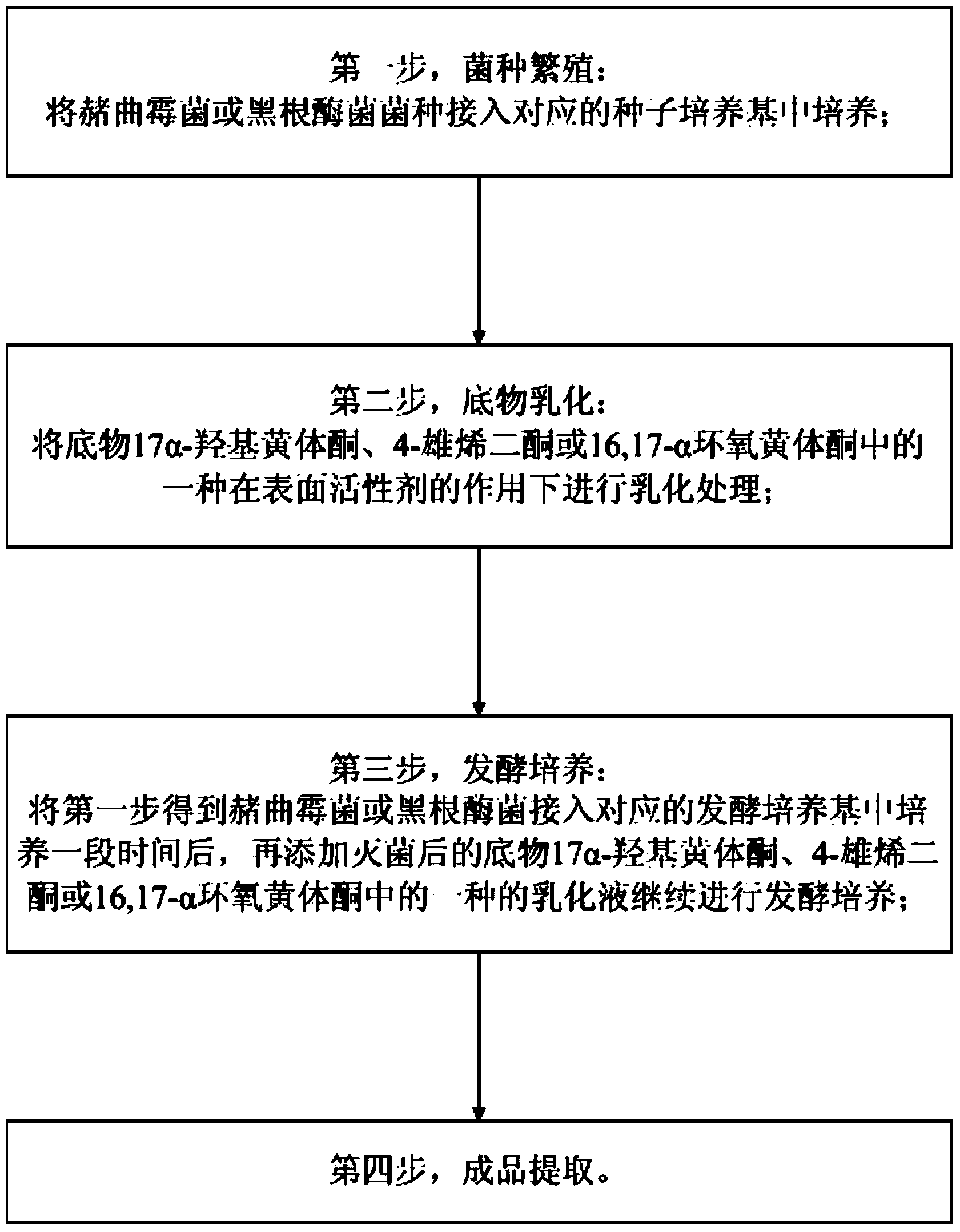
[0030] Such as figure 1 Shown: a preparation method of 11α hydroxylation of an important intermediate of steroid hormones, comprising the following steps:
[0031] The first step, strain propagation:
[0032] Insert the Ochraus ochrax or Nigerizomyces species into the corresponding seed medium for cultivation;
[0033] The second step, substrate emulsification:
[0034] Emulsifying one of the substrates 17α-hydroxyprogesterone, 4-androstenedione or 16,17-αepoxyprogesterone under the action of a surfactant;
[0035] The third step, fermentation culture:
[0036] Put the ochrax or nigeriasis obtained in the first step into the corresponding fermentation medium for a period of time, and then add sterilized 17α-hydroxyprogesterone, 4-androstenedione or 16,17- The emulsion of one of the α-epoxy progesterones continues to be fermented and cultivated;
[0037] The fourth step is to extract the finished product.
[0038] As a preferred technical solution, in another embodiment o...
Embodiment 1
[0053] A method for preparing an important intermediate of steroid hormones by 11α hydroxylation, comprising the following steps:
[0054] The first step, strain propagation:
[0055] Prepare 7 1L eggplant bottle slants of ochrax as a spore suspension and connect them in the seed medium of the seed tank for cultivation, wherein the amount of spores on each eggplant bottle slant is 10 10 -10 12 One, the seed medium includes the following concentrations of components: glucose 25-30g / L, corn steep liquor 20-30g / L, peptone 10-15g / L, yeast extract 5-10g / L, foam enemy 0.03%-0.04% , the pH of the seed medium is 3;
[0056] The second step, substrate emulsification:
[0057] The substrate 17α-hydroxyprogesterone is emulsified under the action of surfactant Tween 80 and temperature 50°C;
[0058] The third step, fermentation culture:
[0059] After 24 hours, transfer the seed culture solution obtained in the first step into the fermentation medium with an inoculation amount of 5% ...
Embodiment 2
[0066] Change the emulsification temperature in Example 1 to 60°C, and the rest of the operations are the same as in Example 1.
[0067] Test results: the conversion rate of 17α-hydroxyprogesterone is 98%, the final titer is 14.37g / L, and the purity of the obtained 11,17α-dihydroxyprogesterone is greater than 98.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com