Novel technology for synthesizing epiandrosterone by adopting 4-androstenedione through selective hydrogenation reducing
A technology of androstenedione and epiandrosterone, which is applied in the field of selective reduction and hydrogenation of 4-androstenedione to synthesize epiandrosterone, can solve the problems of low product purity and yield, high raw material consumption, long reaction route, etc. problems, to achieve the effects of high product purity and yield, shortened reaction routes, and cost savings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
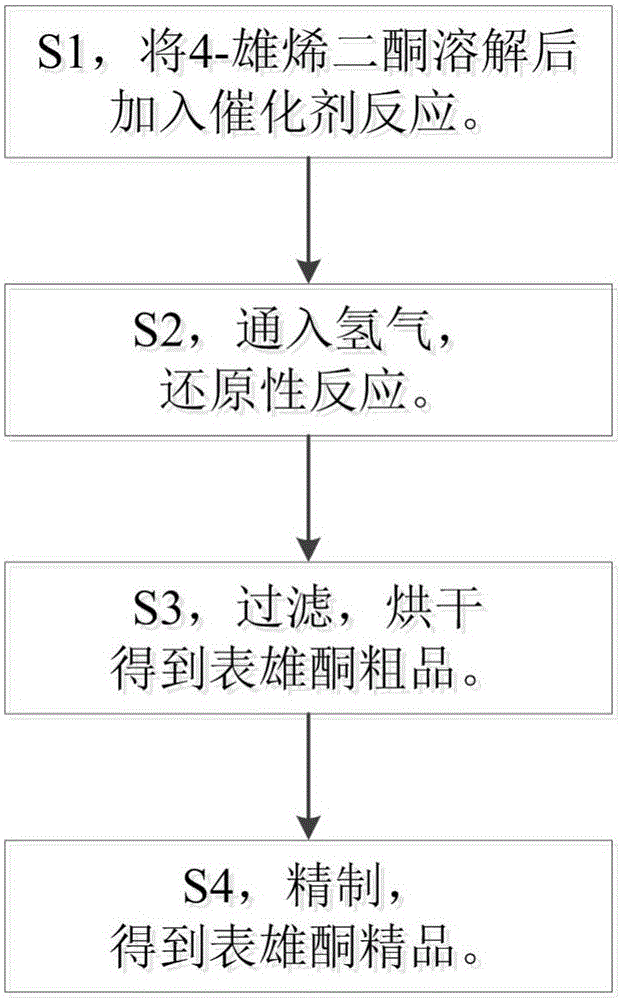
[0024] S1, dissolve 100g of 4-androstenedione in 1.2kg of toluene, heat to dissolve, then cool down to 90°C, add 12.5g of titanium dioxide powder, 37.5g of palladium-calcium catalyst, react for 3 hours, take samples for TLC spot detection until the reaction is complete;
[0025] S2, inject hydrogen gas under 1MP pressure, continue to react for 8 hours, take samples for TLC detection until the reaction is completed;
[0026] S3, after the reaction is completed, filter, recycle titanium dioxide powder and metal platinum, remove toluene by distillation, and dry at 65° C. to obtain crude epiandrosterone;
[0027] S4, take 95 g of the crude epiandrosterone obtained in step S3, dissolve it in 3 times the mass of methanol, add 0.02 times the mass of activated carbon, reflux at 60-70°C for 1 hour, suction filter and concentrate to a paste at 50-55°C, and pump The filtered methanol is recycled, cooled and crystallized at 0°C, and the crystals are filtered and dried to obtain the fine e...
Embodiment 2
[0030] S1, dissolve 100g of 4-androstenedione in 1.2kg of toluene, heat to dissolve, then cool down to 100°C, add 0.5g of titanium dioxide powder, 1g of metal platinum, react for 3 hours, take samples for TLC spot detection until the reaction is complete;
[0031] S2, inject hydrogen gas under 2MP pressure, continue to react for 8 hours, take samples for TLC detection until the reaction is completed;
[0032] S3, after the reaction is completed, filter, recycle titanium dioxide powder and metal platinum, remove toluene by distillation, and dry at 65° C. to obtain crude epiandrosterone;
[0033] S4, taking 95 g of the crude epiandrosterone obtained in step S3, dissolving it in 3 times the mass of methanol, adding 0.02 times the mass of activated carbon, refluxing at 60-70°C for 1.5h, suctioning and concentrating to a paste at 50-55°C, The methanol filtered out by suction is recycled, cooled and crystallized at 5°C, and the crystals are filtered and dried by suction to obtain th...
Embodiment 3
[0037] S1, dissolve 100g of 4-androstenedione in 1.2kg of toluene, heat to dissolve, then cool down to 100°C, add 20g of titanium dioxide powder, 80g of palladium carbon catalyst, react for 5h, take samples for TLC spot detection until the reaction is complete;
[0038] S2, introduce hydrogen gas under 2MP pressure, continue to react for 10h, take samples for TLC detection until the reaction is completed;
[0039] S3, after the reaction is completed, filter, recycle the titanium dioxide powder and platinum metal, distill to remove toluene, and dry at 95° C. to obtain crude epiandrosterone;
[0040] S4, take 95 g of the crude epiandrosterone obtained in step S3, dissolve in 3 times the mass of methanol, add 0.02 times the mass of activated carbon, reflux at 60-70°C for 2 hours, suction filter and concentrate to a paste at 50-55°C, and pump The filtered methanol is recycled, cooled and crystallized at 5°C, and the crystals are filtered and dried to obtain the fine epiandrosteron...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com