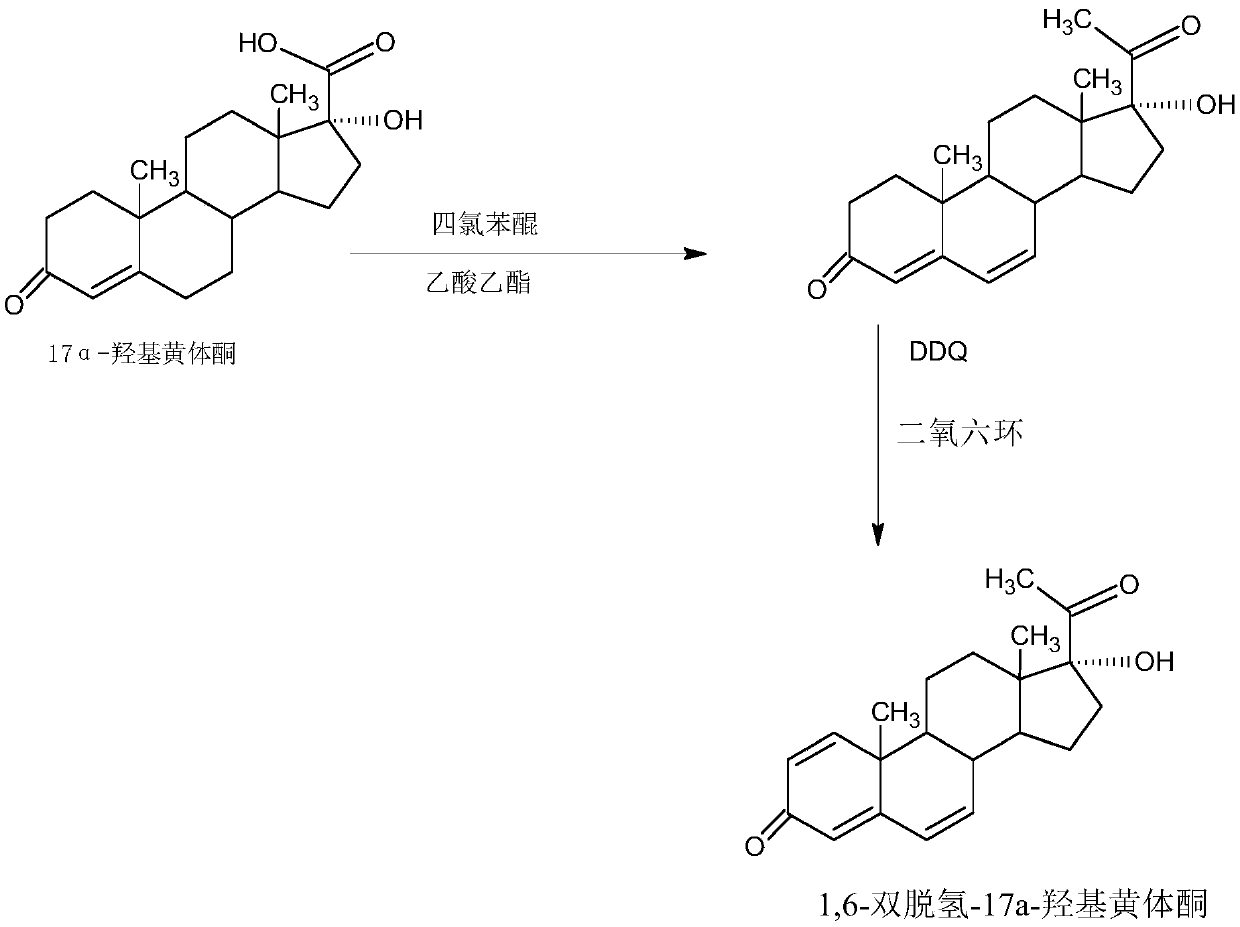
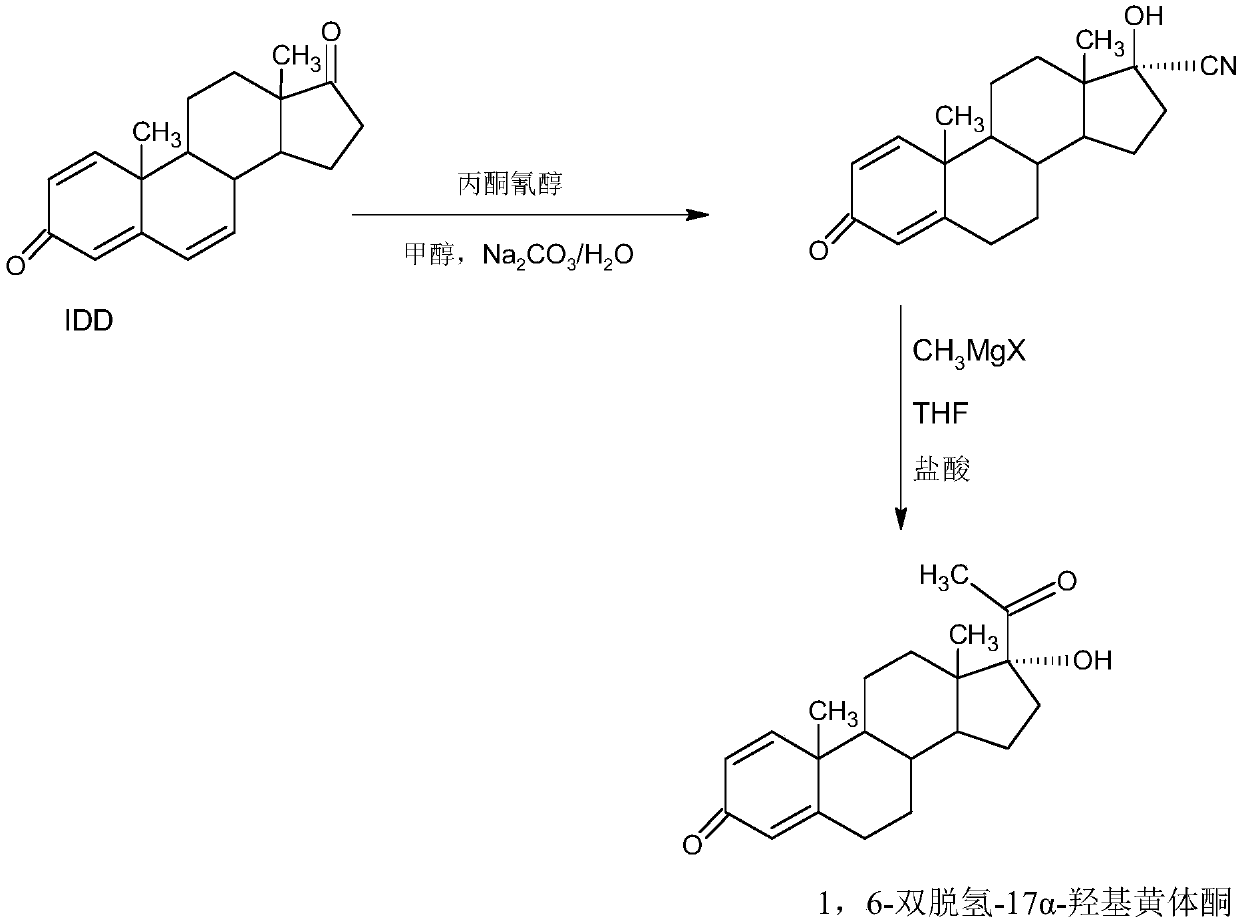
Preparation method of 1,6-didehydrogenation-17a-hydroxyl progesterone product
A technology of hydroxyprogesterone and double dehydrogenation, applied in the direction of steroids, organic chemistry, etc., can solve the problems of complicated production process operation, rising production costs, and increased production costs, and achieve simple and environmentally friendly processes, reduced production costs, and high quality Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A: Preparation of Hydroxycyanide
[0040] In a 1000ml three-neck flask, add 100g of starting material IDD and 250ml of acetone cyanohydrin, control the temperature to 40-45°C, stir to completely dissolve IDD, then control the temperature at 20-25°C, and slowly add 2% carbonic acid dropwise Sodium alkali aqueous solution, about 2-2.5 hours to drop, then keep warm at 25-30°C for 2-3 hours, TLC to confirm the reaction end point, after the reaction, concentrate under reduced pressure to recover 90-95% of the solvent acetone cyanohydrin, and the remaining Thing is cooled to 20~25 ℃, then adds 500ml tap water water analysis, filters, and filtrate is sent to waste water treatment station for processing, and filter cake is crystallized with 500ml 40% alcohol aqueous solution, obtains hydroxycyanide 98.6g, HPLC content 98.2%, weight yield 98.6%.
[0041] B: Preparation of 1,6-didehydro-17a-hydroxyprogesterone
[0042] In a 1000ml three-neck flask, add 35g of magnesium powder a...
Embodiment 2
[0045] A: Preparation of Hydroxycyanide
[0046] In a 1000ml three-neck flask, add 100g of starting material IDD, 50ml of acetone cyanohydrin, and 500ml of methanol, control the temperature to 40-45°C, stir to completely dissolve IDD, then control the temperature at 20-25°C, slowly add 2 % sodium hydroxide alkaline aqueous solution, drop it in about 2-2.5 hours, then keep warm at 25-30°C for 2-3 hours, TLC confirms the reaction end point, after the reaction, concentrate under reduced pressure to recover 90-95% methanol and acetone The mixed solvent of cyanohydrin was applied mechanically, the residue was cooled to 20-25° C., and then 500 ml of tap water was added for water analysis, filtered, and the filtrate was sent to the waste water treatment station for treatment. The filter cake was crystallized with 500 ml of 40% alcohol aqueous solution to obtain 97.8 g of hydroxycyanide. HPLC content 98.5%, weight yield 97.8%.
[0047] B: Preparation of 1,6-didehydro-17a-hydroxyproge...
Embodiment 3
[0051] A: Preparation of Hydroxycyanide
[0052] In a 1000ml three-necked flask, add 100g of starting materials IDD, 50ml of acetone cyanohydrin, and 500ml of DME, control the temperature to 40-45°C, stir to completely dissolve IDD, then control the temperature at 20-25°C, and slowly add 2% Triethylamine alkali aqueous solution, drop it in about 2-2.5 hours, then keep warm at 25-30°C for 2-3 hours, TLC confirms the reaction end point, after the reaction, concentrate under reduced pressure to recover 90-95% of DME and acetone cyanide The mixed solvent of alcohol is used mechanically, the residue is cooled to 20-25°C, and then 500ml of tap water is added for water analysis, filtered, and the filtrate is sent to the waste water treatment station for treatment. The filter cake is crystallized with 500ml of 40% alcohol aqueous solution to obtain 96.2g of hydroxycyanide, HPLC Content 98.2%, weight yield 96.2%.
[0053] B: Preparation of 1,6-didehydro-17a-hydroxyprogesterone
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com