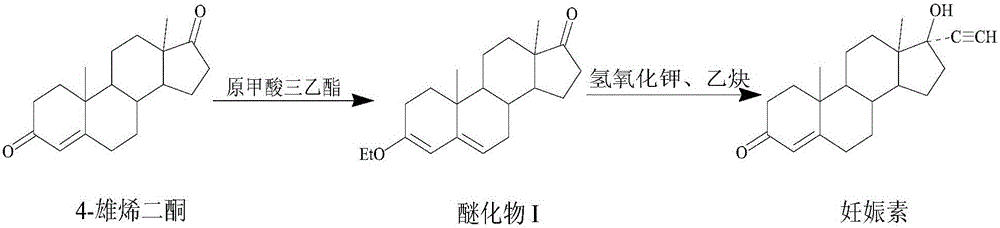
Synthetic method for ethisterone
A synthesis method and a progestogen technology are applied in the directions of steroids, organic chemistry, etc., can solve the problems of increased labor cost, increased progesterone synthesis cost, etc., and achieve the effects of simple operation, solving incomplete reaction, and improving catalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
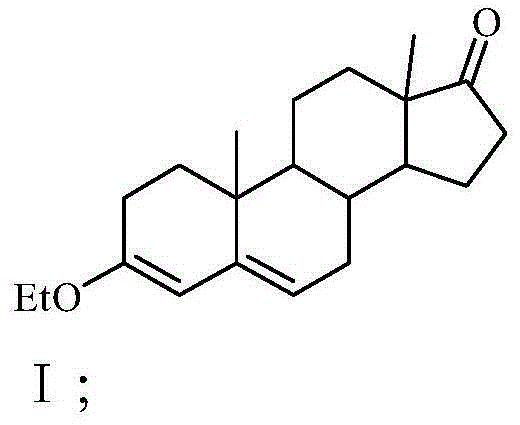
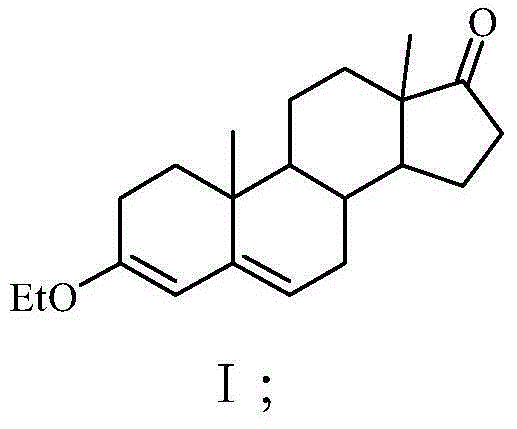
[0028] Step 1. Add 70L of absolute ethanol and 66kg of 4-androstenedione to a clean 200L etherification kettle. After stirring evenly, add 41L of triethyl orthoformate and 1.12kg of pyridine hydrobromide into the etherification kettle After the temperature was raised to 40°C, the etherification reaction was carried out under heat preservation and stirring for 3.5 hours, and there was no raw material point for sampling chromatography. After the reaction material after the etherification reaction was cooled to room temperature, 1.98 L of triethylamine was added to the reaction material, stirred and neutralized The pH value of the final reaction material is 7, and then the reaction material with a pH value of 7 is cooled to below 5°C, and the crystals are obtained by filtration, and the crystals are washed with a mixed solution of triethylamine and absolute ethanol. Dry below ℃ to constant weight to obtain 65kg ether compound I with a yield of 98.48%; the volume percentage of trie...
Embodiment 2
[0036]Step 1. Add 70L of absolute ethanol and 66kg of 4-androstenedione to a clean 200L etherification kettle, stir evenly, and then add 38L of triethyl orthoformate and 0.99kg of pyridine hydrobromide into the etherification kettle After the temperature was raised to 35°C, the etherification reaction was carried out under heat preservation and stirring for 5 hours, and there was no raw material point for sampling chromatography. After the reaction material after the etherification reaction was cooled to room temperature, 1.75L triethylamine was added to the reaction material, and after stirring and neutralization The pH value of the reaction material is 6, and then the reaction material with a pH value of 6 is cooled to below 5°C, and the crystals are obtained by filtration, the crystals are washed with a mixed solution of triethylamine and absolute ethanol, and after drying, the Following drying to constant weight, 64.5kg ether compound I was obtained with a yield of 97.73%; ...
Embodiment 3
[0044] Step 1. Add 70L of absolute ethanol and 66kg of 4-androstenedione to a clean 200L etherification kettle, stir well, and then add 46L of triethyl orthoformate and 1.98kg of pyridine hydrobromide into the etherification kettle After the temperature was raised to 45°C, the etherification reaction was carried out under the condition of heat preservation and stirring for 3 hours, and there was no raw material point for sampling chromatography. After the reaction material after the etherification reaction was cooled to room temperature, 2.12L triethylamine was added to the reaction material, and after neutralization by stirring The pH value of the reaction material is 8, and then the reaction material with a pH value of 8 is cooled to below 5°C, and the crystals are obtained by filtration, and the crystals are washed with a mixed solution of triethylamine and absolute ethanol, and after being dried, they are stored at 80°C Following drying to constant weight, 64.8kg ether comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com