Synthesis technology of finasteride
A technology of finasteride and synthesis technology, applied in the field of synthesis technology of finasteride, can solve the problems of many side reactions, expensive reagents, many by-products and the like, and achieves the effects of easy operation, reduced production cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
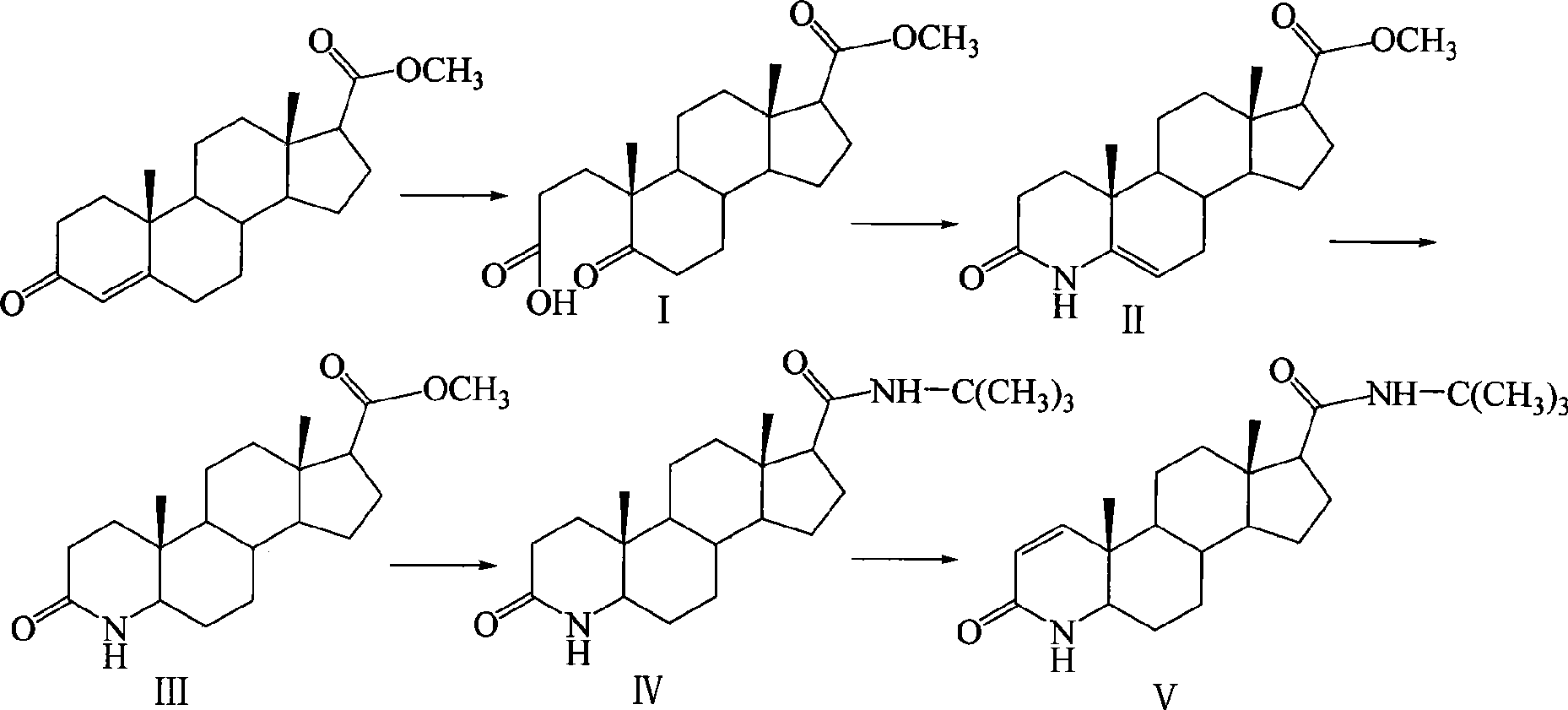
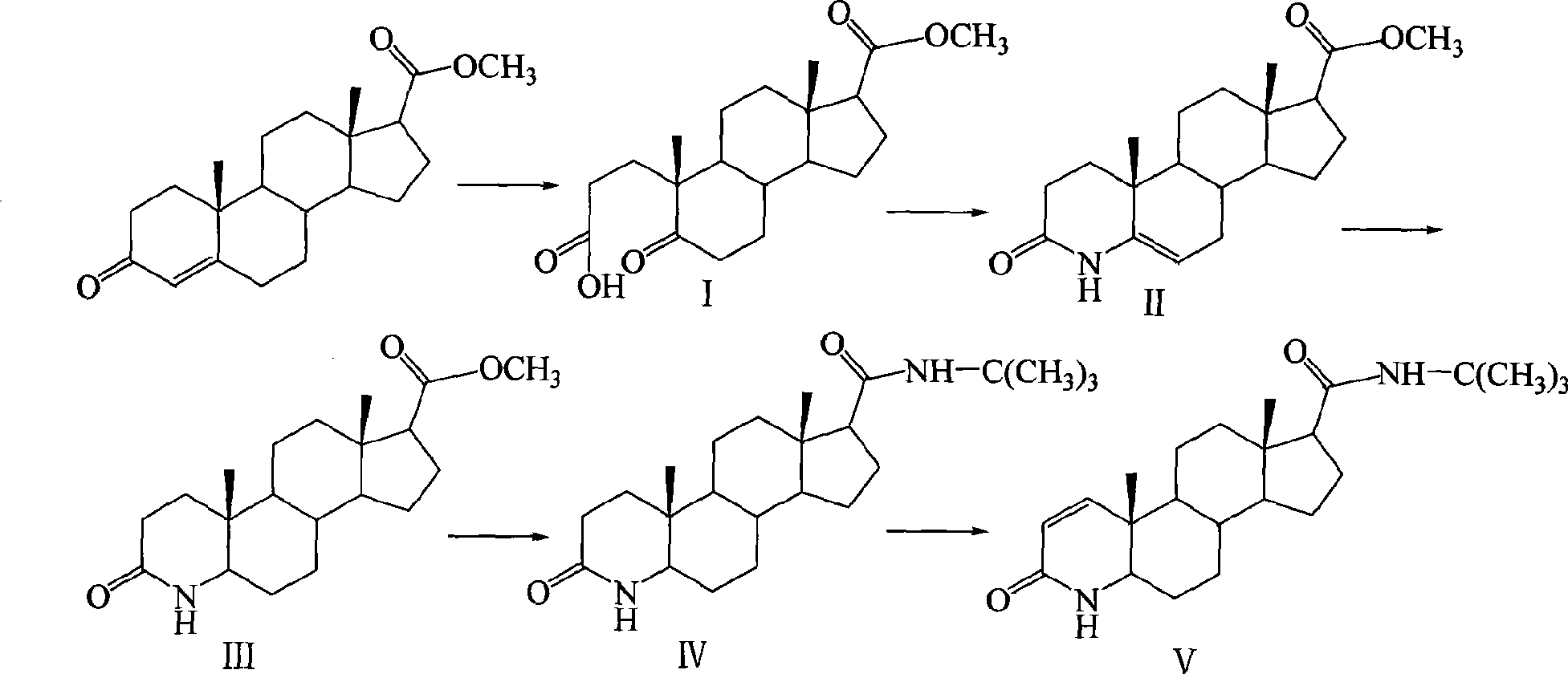
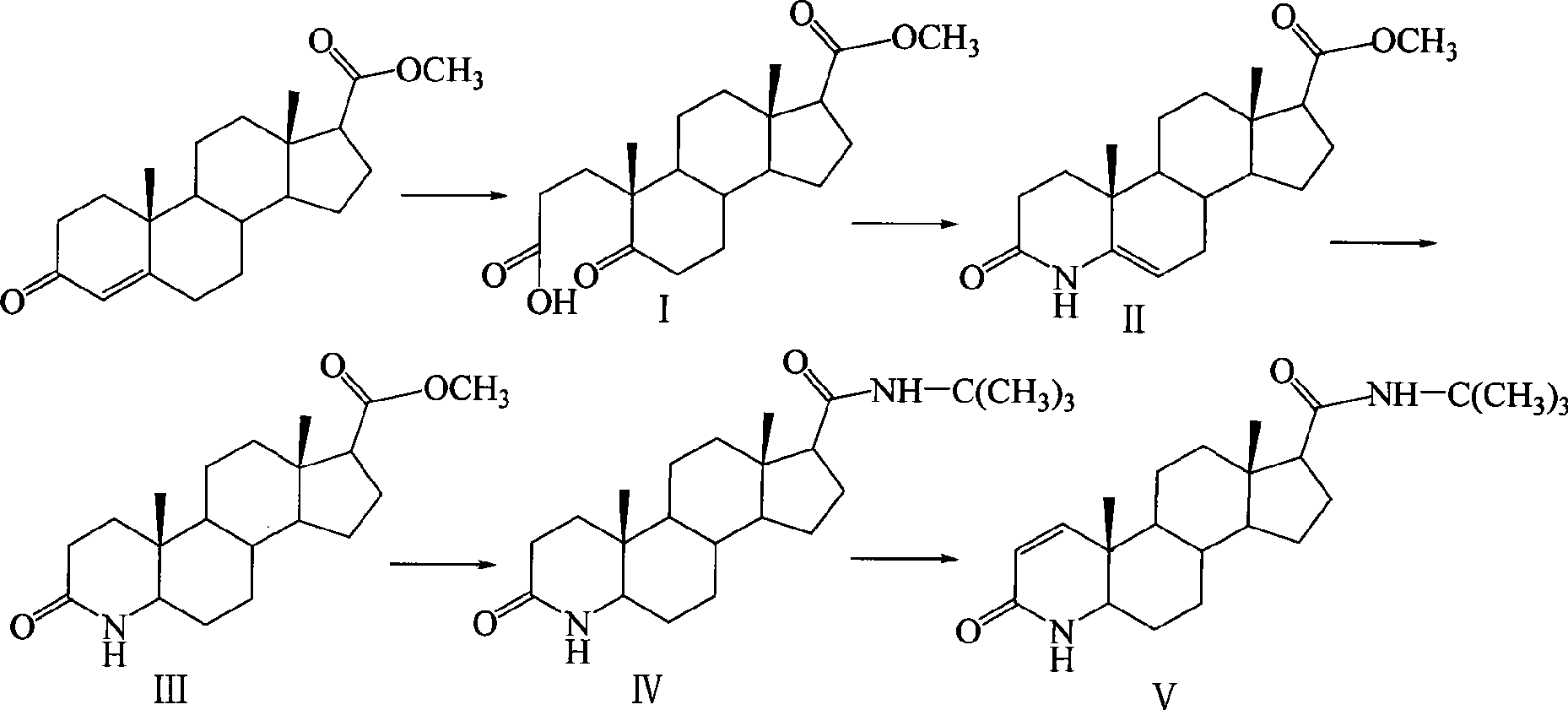
[0027] The synthetic route is as follows:
[0028]
[0029] The first step, the preparation of 5-carbonyl-17β-carboxylate methyl ester-A-abortion-3,5-cleavage-androst-3-acid (I)
[0030] 200mL of tert-butanol, 10g (31.6mmol) of methyl 3-carbonyl-4-androstene-17β-carboxylate, add a solution of 10g (94.3mmol) of anhydrous sodium carbonate dissolved in 30mL of water under stirring, and add dropwise under reflux A solution of 40g (22.4mmol) of sodium periodate and 390mg (2.47mmol) of potassium permanganate dissolved in 200mL of water was added, then refluxed for 30min, cooled to room temperature, filtered, most of the tert-butanol was evaporated under reduced pressure, iced Adjust the pH to 2 with 6N hydrochloric acid under bath cooling, extract with ethyl acetate, combine the organic layers, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain a transparent oil, add a small amount of petroleum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com