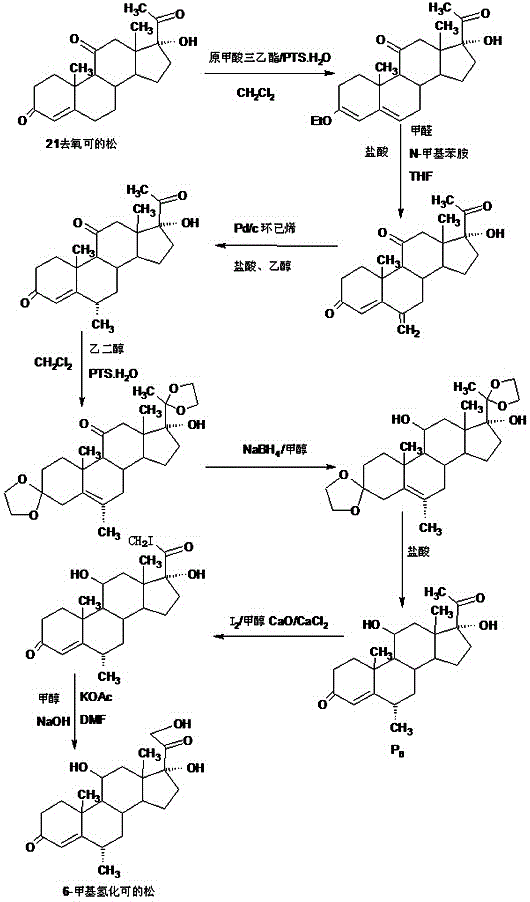
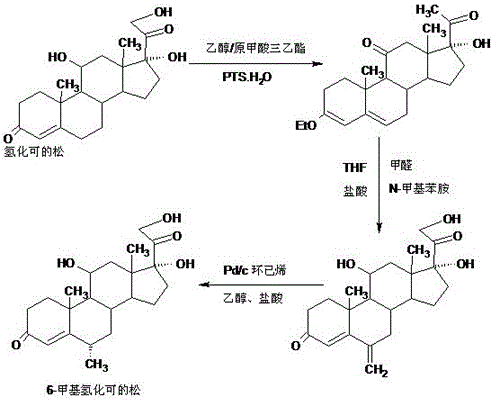
Preparation method of 6a-methyl hydrocortisone
A technology of methyl hydrocortisone and hydrocortisone, which is applied in directions such as organic chemistry, steroids, etc., can solve the problems of increased production cost, long synthesis route, low total synthesis yield, etc., and achieves reduced production cost, The production operation is simple, the process is economical and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A. Preparation of ether compounds
[0028] In a 1000ml three-neck flask, add 100g hydrocortisone, 200ml ethanol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 40-45 degrees and stir for 6-8 hours, TLC detects the end point of the reaction, the reaction is complete Finally, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then recover 90-95% organic solvent under reduced pressure, then add 500ml of 50% ethanol aqueous solution, cool the system to -5-0 degrees, stir and crystallize for 2~ After 3 hours, filter with suction, wash with a small amount of ethanol aqueous solution, combine the lotion and filtrate, recover the solvent, and dry the filter cake below 70°C to obtain 101.6 g of ether compound, the HPLC content is 99.2%, and the weight yield is 101.6%.
[0029] B. Preparation of methine
[0030]In a 1000ml three-necked flask, add 100g of ether compound, 800ml of ethanol, and 50ml of dichloromethane. After dissolving, add a s...
example 2
[0034] A, the preparation of ether compound
[0035] In a 1000ml three-neck flask, add 100g hydrocortisone, 200ml chloroform, 80ml triethyl orthoformate, 2g concentrated sulfuric acid, keep warm at 40-45 degrees and stir for 6-8 hours. TLC detects the end point of the reaction. After the reaction, Add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then recover 90-95% organic solvent under reduced pressure, then add 500ml of 50% ethanol aqueous solution, cool the system to -5-0 degrees, stir and crystallize for 2-3 hours , suction filtration, washed with a small amount of ethanol aqueous solution, the washing liquid and the filtrate were combined, the solvent was recovered, and the filter cake was dried below 70 degrees to obtain 101.2 g of ether compound, the HPLC content was 99.0%, and the weight yield was 101.2%.
[0036] B. Preparation of methine
[0037] In a 1000ml three-necked flask, add 100g of ether compound, 800ml of tetrahydrofuran, and 50ml of chlo...
Embodiment 3
[0041] A, the preparation of ether compound
[0042] In a 1000ml three-neck flask, add a mixed solution of 100g hydrocortisone, 200ml toluene, 80ml triethyl orthoformate, 2g HCl and 20ml ethanol, keep warm at 40-45 degrees and stir for 6-8 hours, TLC detects the reaction end point, After the reaction, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then recover 90-95% of the organic solvent under reduced pressure, then add 500ml of 50% ethanol aqueous solution, cool the system to -5-0 degrees, stir and crystallize After 2 to 3 hours, filter with suction, wash with a small amount of ethanol aqueous solution, combine the lotion and filtrate, recover the solvent, and dry the filter cake below 70 degrees to obtain 100.8 g of ether compound, with an HPLC content of 99.5% and a weight yield of 100.8%.
[0043] B. Preparation of methine
[0044] In a 1000ml three-neck flask, add 100g of ether compound, 800ml of toluene, and 50ml of chloroform, and after dissolvin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com