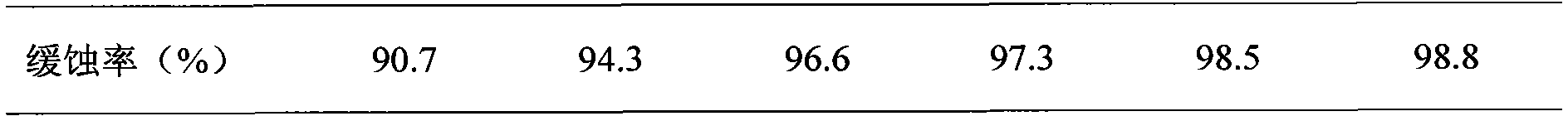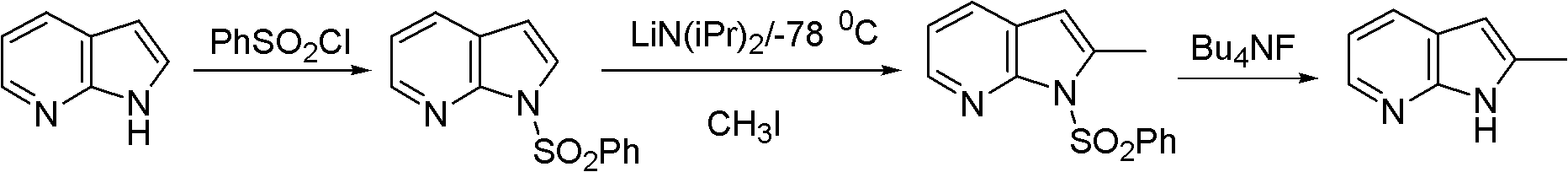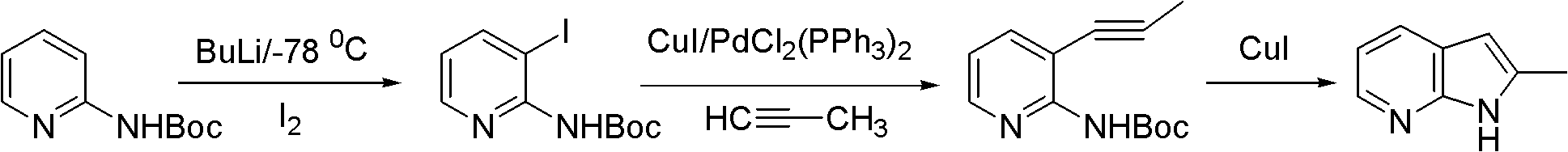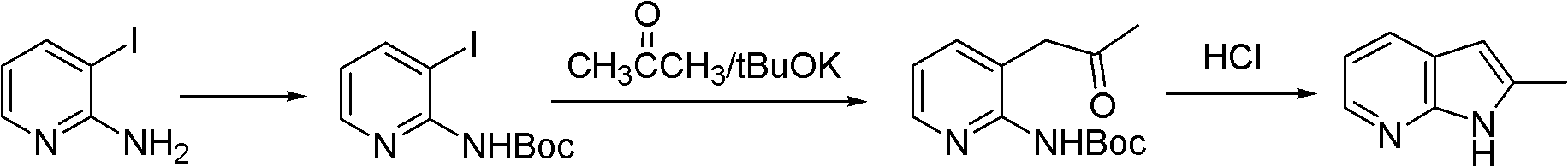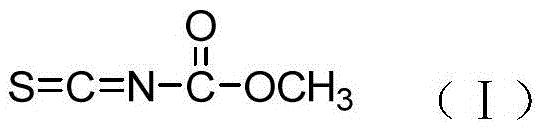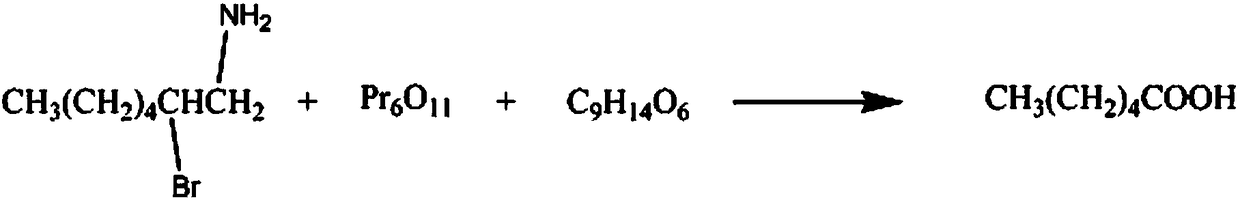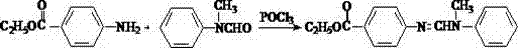Patents
Literature
54 results about "N-Methylaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
N-Methylaniline (NMA) is an aniline derivative. It is an organic compound with the chemical formula C₆H₅NH(CH₃). The substance is a colorless viscous liquid, Samples turn brown when exposed to air. The chemical is insoluble in water. It is used as a latent and coupling solvent and is also used as an intermediate for dyes, agrochemicals and other organic products manufacturing. NMA is toxic and exposure can cause damage to the central nervous system and can also cause liver and kidney failure.
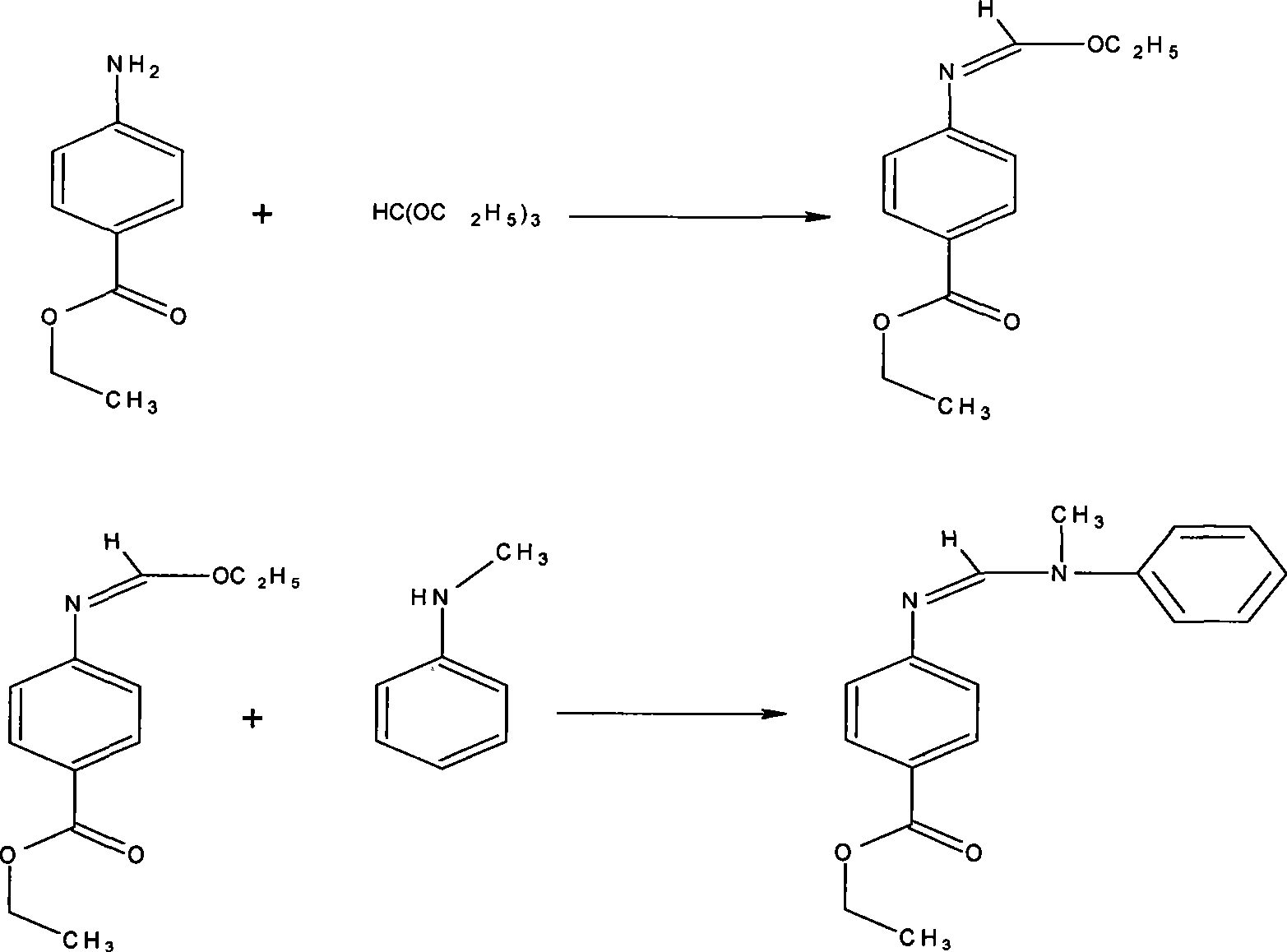
Preparation of N-(4-ethoxy carbonyl phenyl)-N'-methyl-N'-phenyl formamidine
ActiveCN101481330AReduce consumptionLower the condensation reaction temperatureOrganic chemistryN-MethylanilineEthyl aminobenzoate
The invention relates to the technical field of fine chemistry industry, in particular to a method for preparing N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenylformamidine, comprising the following two condensation reaction processes: in the first condensation reaction process, parathesin is mixed with trialkyl ortho-formate according to the mass ratio of 1:1-1:10 for the condensation reaction at the temperature of 100-200 DEG C and reduced pressure distilling is carried out to obtain intermediate; in the second condensation reaction process, the condensation reaction is carried out on the intermediate generated in the first condensation reaction process and N-methylaniline according to the mass ratio of 1:1-1:5 at the temperature of 100-200 DEG C and simple reduced pressure distilling is carried out on reaction liquid to obtain the N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenylformamidine. The method solves the problem of difficulty in large-scale industrial production in the prior art.
Owner:CHANGZHOU SUNLIGHT PHARMA
Method for preparing N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine
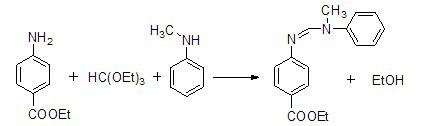
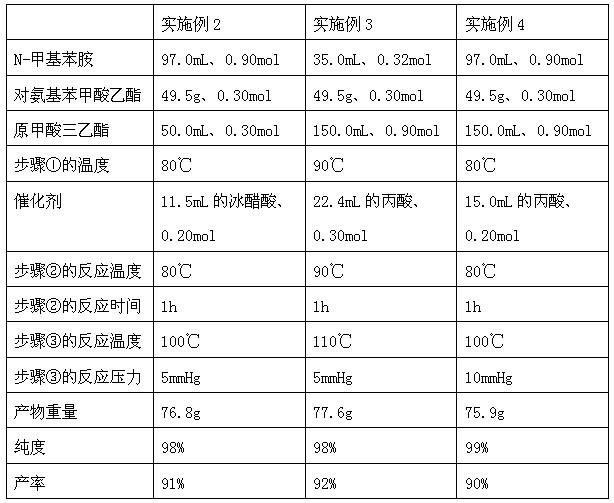
The invention discloses a method for preparing N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine. The method comprises the following steps of: (1) adding N-methylaniline, ethyl p-aminobenzoate and triethyl orthoformate into a reaction device, stirring and heating up to 80-90 DEG C; (2) adding propionic acid or glacial acetic acid used as a catalyst into the reaction device, meanwhile, evaporating out generated alcohol, and reacting for 1-3 hours at a temperature of 80-90 DEG C; (3) heating up to 100-110 DEG C, meanwhile, reducing the pressure to 1-20mmHg, continuously reacting until no alcohol is evaporated out; and (4) after the reaction ends, reducing the pressure and distilling to obtain the N-(4-ethyoxylcarbonylphenyl)-N'-methyl-N'-phenyl carbonamidine. The method has the advantages of simple process, moderate condition and high productivity and is suitable for large-scale industrial production.
Owner:大连新阳光材料科技有限公司 +1
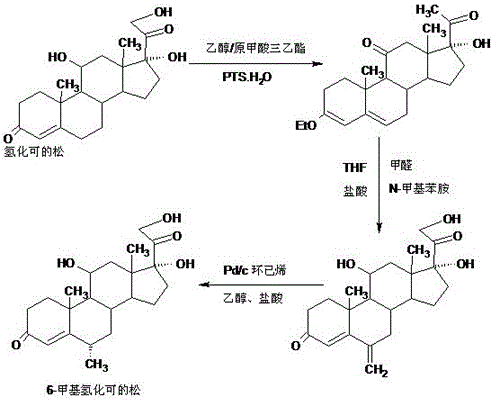
Preparation method of 6a-methyl hydrocortisone
InactiveCN106518945AWide variety of sourcesProcess economy and environmental protectionSteroidsSolventMethyl group
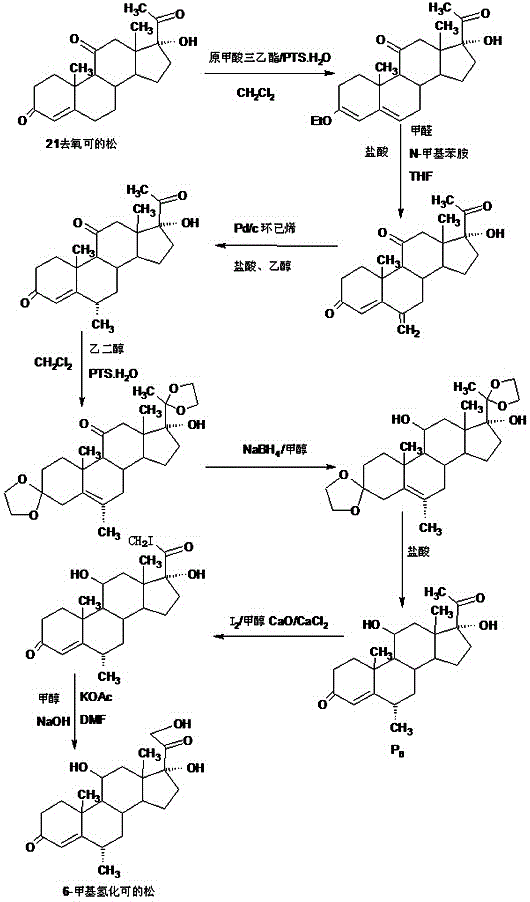
The invention provides a preparation method of 6a-methyl hydrocortisone. The preparation method comprises the steps that hydrocortisone prepared from 4-androstene-3,17-dione (called as 4AD for short) is adopted as a raw material to generate an acid catalytic reaction with triethyl orthoformate in an organic solvent, and etherate 3-ether enol hydrocortisone is obtained; the etherate generates a Manlixi reaction with N-methylaniline and formaldehyde in an organic solvent, and a methylene product 6-methylene hydrocortisone is obtained; the methylene product generates a catalytic hydrogenation reaction in an organic solvent, and 6a-methyl hydrocortisone is obtained. Compared with a production method achieved by taking a mold removal product obtained by processing diosgenin as a raw material, the method for producing 6a-methyl hydrocortisone has the advantages that raw material sources are wide, the processes are economical and environmentally friendly, production operation is easy and convenient, the synthetic route is short, and the product yield is high; by producing 6a-methyl hydrocortisone through the method, the production cost is reduced by 40%-50% compared with a traditional method; the solvents used in production can be recycled and cyclically reused, and implementation of industrialized production is promoted.
Owner:HUNAN KEREY BIOTECH
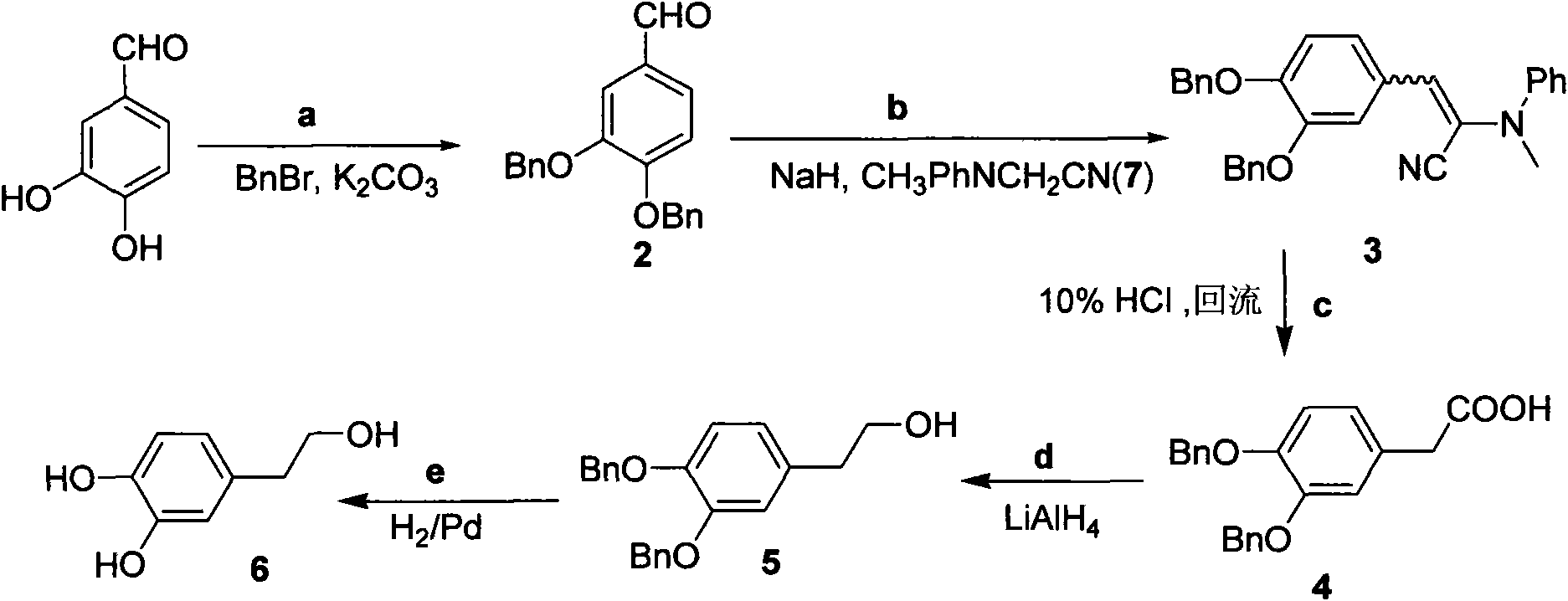
Method for preparing hydroxytyrosol
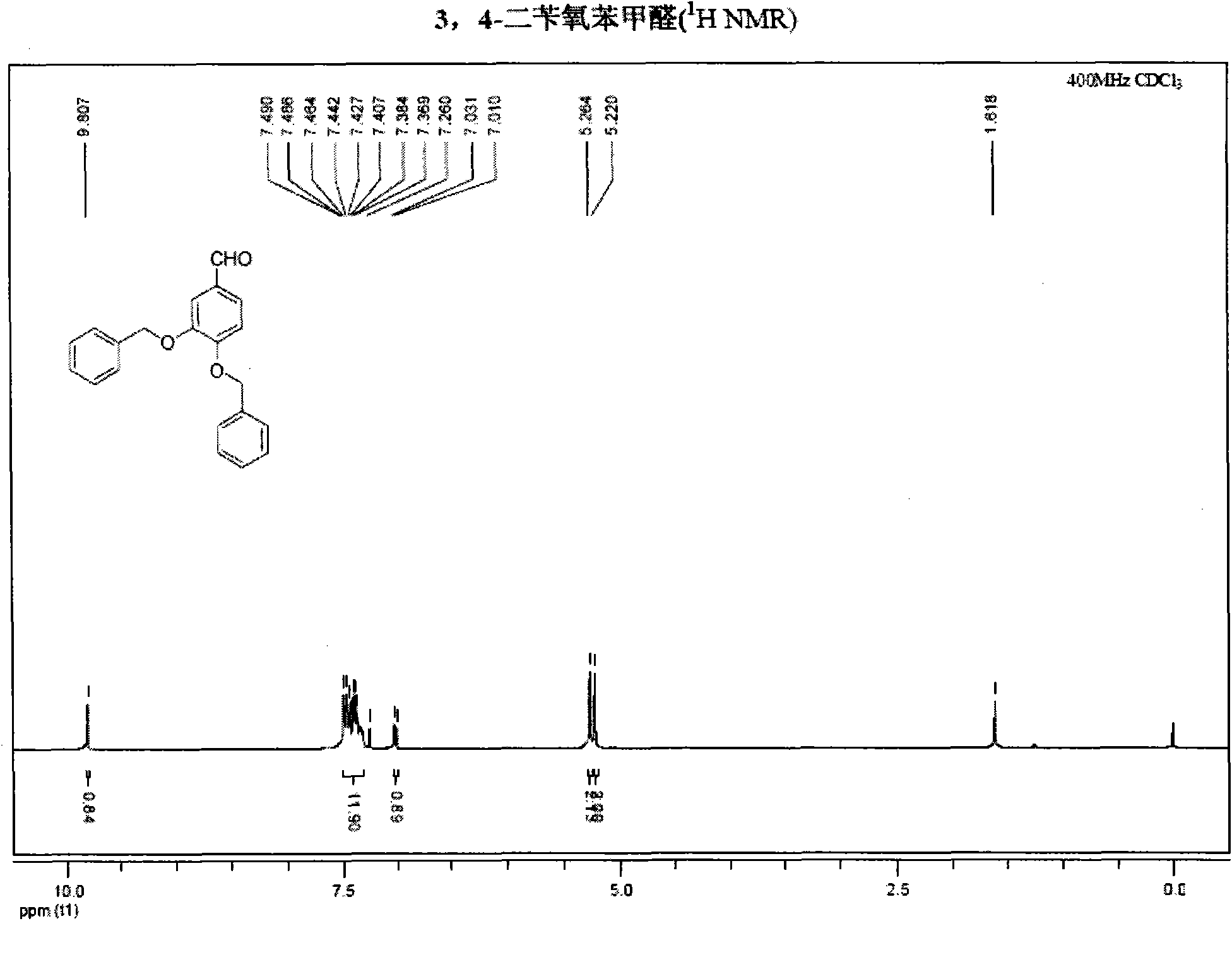
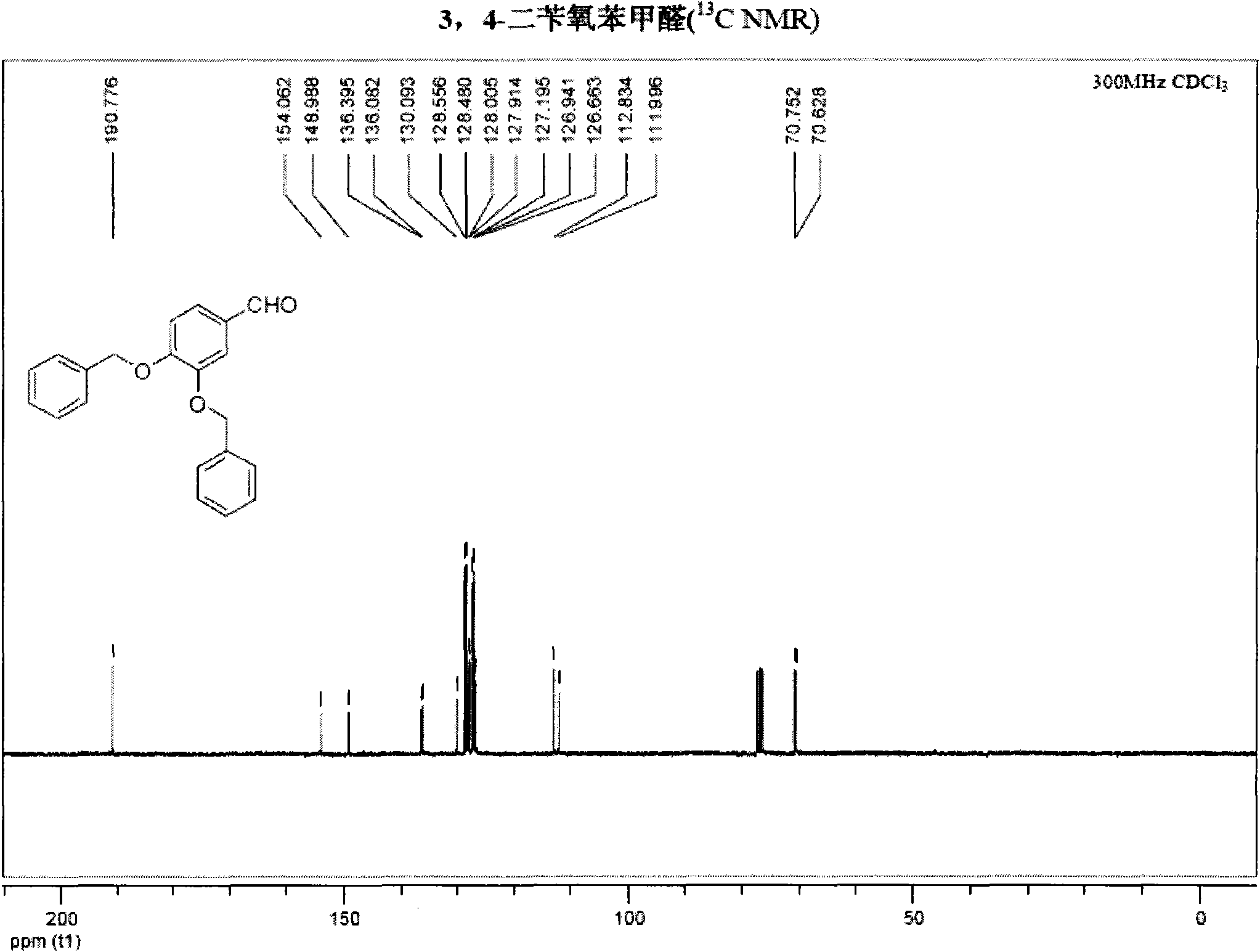
InactiveCN101891595AMild reaction conditionsFew reaction stepsOrganic chemistryOrganic compound preparationHydroxytyrosolBenzoyl bromide
The invention belongs to the field of medicinal synthesis, and particularly relates to a method for preparing hydroxytyrosol, which comprises the following steps of: (1) protecting free hydroxyl groups at 3 and 4 positions, on 3,4-dihydroxy benzaldehyde with benzyl, namely, reacting the 3,4-dihydroxy benzaldehyde with benzyl bromide to prepare 3,4-dibenzyloxybenzaldehyde; (2) reacting N-methylaniline acetonitrile with the 3,4-dibenzyloxybenzaldehyde to prepare 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile, and hydrolyzing the 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile under acidic condition to prepare a 3,4-dibenzylosyphenylacetic acid; (3) reducing a carboxyl group of the 3,4-dibenzylosyphenylacetic acid with lithium borohydride, lithium aluminum hydride or sodium borohydride to prepare 3,4-dibenzyloxyphenethyl alcohol; and (4) catalyzing the 3,4-dibenzyloxyphenethyl alcohol with a catalyst palladium / carbon to prepare the hydroxytyrosol. The reagents used in the method are easily obtainable and low in cost, reaction conditions are mild, and the final overall yield of the whole reaction reaches 50 to 60 percent.
Owner:SUZHOU UNIV
Nitrocellulose Composition And Uses Therefor
InactiveUS20090199938A1Easy to burnNitrocellulose explosive compositionsNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsNitrocelluloseMethyl group
A nitrocellulose composition comprises an admixture comprising: from about 75 weight percent to about 85 weight percent nitrocellulose; and from about 15 weight percent to about 25 weight percent camphor, based upon the weight of the admixture; and from about 1 weight percent to about 5 weight percent of a stabilizer (based on the total weight of the composition), wherein the stabilizer is chosen from the group consisting of N,N-Diethyl-N,N′-diphenylurea, N,N′-Dimethyl-N,N′-diphenylurea, 1,1-Diphenylurea, N-methyl-N,N′-diphenylurea, 1-Ethyl-3,3′-diphenylurea, Diphenylamine, 2-Nitro-diphenylamine, 4-Nitro-diphenylamine; Triphenylamine; p-Nitro-N-methylaniline, p-Nitro-ethylaniline, soybean oil, castor oil, sodium silicate, lactic acid amide, and benzonate; and from about 1 weight percent to about 5 weight percent of azodicarbonic acid diamide, based on the total weight of the composition. The composition is heated at a temperature sufficient to cause it to polymerize, after which it is formed into sheets, from which components for munitions, such as artillery, small arms, modular artillery charge systems and mortar increments are formed. The components of the mortar increments are joined together, and after being filled with a propellant composition, one or more mortar increments is attached to a mortar shell.
Owner:KAUFMANN & GOTTWALD ZELLULOID & PLASTIKWARENFAB
Method for synthesizing thiazine ketone
The invention discloses a method for preparing buprofezin, which adopts benzene, chlorobenzene orhomologue as the solvent during the photochemical chlorination producing process of buprofezin to replace carbon tetrachloride used in the prior art. The phosgenation, chlorination and desolvation operations are performed on N-methylaniline to get the solution with the N-chloromethyl-phenyl-amido-formyl chloride. The invention has the advantages that average photochemical chlorination yield of the N-methylaniline is more than 80%, and buprofezin content in the finished product is more than 95.0%; benzene, chlorobenzene or homologue can entirely replace carbon tetrachloride to process industrial buprofezin production so as to avoid the environment pollution.
Owner:JIANGSU ANPON ELECTROCHEM
Method for synthesizing (+/-)-9-O-demethyl-alpha-dihydrotetrabenazine
ActiveCN101985446AThe steps are well designedSimple and fast operationOrganic chemistryDiethyl etherMethyl group
The invention discloses a method for synthesizing (+ / -)-9-O-demethyl-alpha-dihydrotetrabenazine, which comprises the following steps of: adding sodium hydride and hexamethylphosphoramide into dimethylbenzene, heating to the temperature of between 90 and 100 DEG C, dripping N-methylaniline, and stirring for 15 to 20 minutes; dripping dimethylbenzene suspension of (+ / -)-alpha-dihydrotetrabenazine into the mixed solution, after dripping, continuously stirring the reaction suspension at the temperature of between 90 and 100 DEG C for 48 to 50 hours; slowly adding aqueous solution of sodium hydroxide into the reaction suspension, separating out an aqueous phase, neutralizing the aqueous phase by using aqueous solution of hydrochloric acid until the pH value is 7 to 9, extracting by using diethyl ether, concentrating an organic phase under reduced pressure to obtain a light brown solid; and purifying the light brown solid by using column chromatography technology to obtain the target product. By optimizing the reaction conditions of the prior art and improving posttreatment and purification technology, the method has the advantages of low preparation cost, high repeatability and high yield.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Anti-coking energy-saving agent for coal-fired boiler
InactiveCN104745270AIdeal decokingIdeal anti-focus effectFuel additivesN-MethylanilineCalcium hydroxide
The invention relates to the technical field of the fine chemical engineering, and concretely relates to an anti-coking energy-saving agent for a coal-fired boiler. The anti-coking energy-saving agent for coal-fired boiler comprises, by weight, 15-28 parts of calcium formate, 15-23 parts of potassium permanganate, 10-18 parts of calcium hydroxide slag, 3-5 parts of magnesium chloride, 8-12 parts of silica, 3-4 parts of quartz powder and 1-3 parts of N-methylaniline. The anti-coking energy-saving agent for coal-fired boiler, provided by the invention, has the advantages of ideal effect and manpower saving.
Owner:吴旭
Preparation method of buprofezin
PendingCN108530388AReduce generationImprove protectionOrganic chemistrySodium bicarbonateChlorobenzene
The invention provides a preparation method of buprofezin, comprising the steps of 1), adding sodium thiocyanate and water into an esterification kettle until dissolution; adding tertiary butanol andhydrochloric acid to obtain a mixed ester; allowing transposition and catalytic reaction to obtain t-butyl isothiocyanate; 2), adding the t-butyl isothiocyanate into chlorobenzene, stirring, dropwiseadding isopropyl amine to obtain 1-isopropyl-3-tert-butylthiourea solution; 3), adding N-methylaniline into chlorobenzene; introducing carbonyl chloride and chlorine gas in sequence to obtain N-chloromethyl-N-benzenecarbamoylchloride solution; 4), adding sodium bicarbonate into water, and adding the resultant into chlorobenzene; adding the 1-isopropyl-3-tert-butylthiourea solution; dropwise addingthe N-chloromethyl-N-benzenecarbamoylchloride solution, filtering, allowing layering, performing high-vacuum steaming to remove the chlorobenzene, crystallizing, centrifuging, and drying to obtain buprofezin. The substitutive average yield of the preparation process of buprofezin reaches 90% and above; the content of finished buprofezin reaches 98.0% and above; the preparation process is free ofammonia nitrogen wastewater.
Owner:JIANGSU ANPON ELECTROCHEM
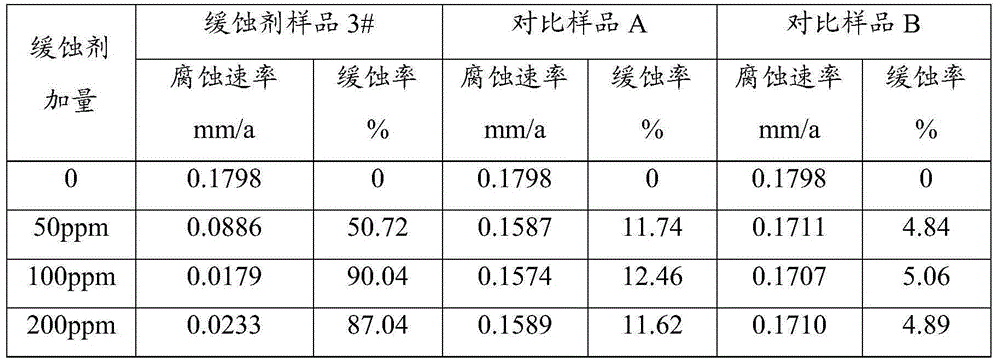
Corrosion inhibitor and preparation method thereof
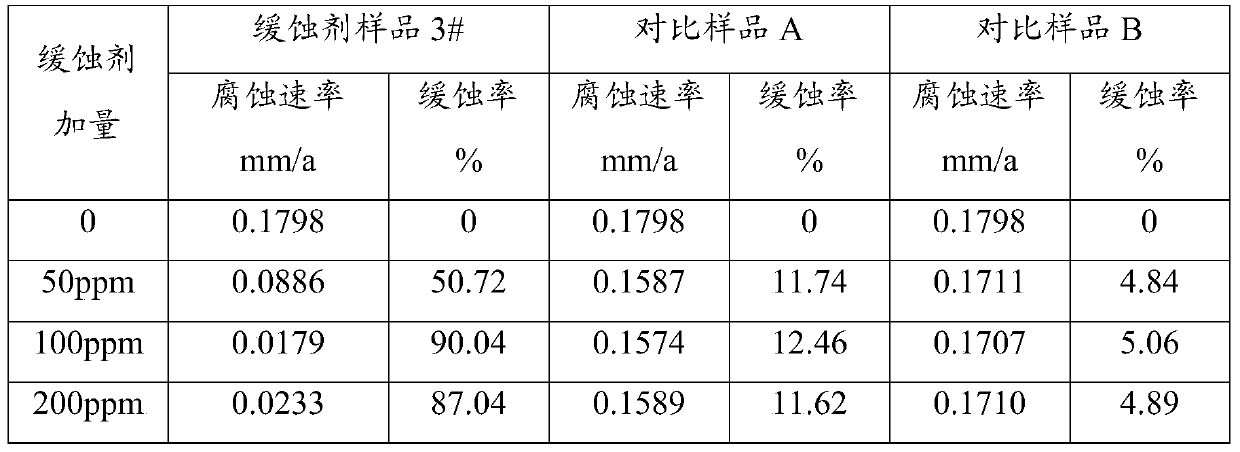
ActiveCN105154049BCorrosion controlDrilling compositionBorehole/well accessoriesN-MethylanilineSodium molybdate
The invention relates to a corrosion inhibitor and a preparation method thereof. The corrosion inhibitor comprises, by weight: 50-70% of Mannich base, 10-20% of phosphate, 10-20% of isopropanol, and 10-20% of sodium molybdate. The preparation method of the corrosion inhibitor comprises: adding N-methylaniline into a three-neck flask, heating the flask to 80 DEG C, and adjusting the pH value to 2 with concentrated hydrochloric acid; heating the flask to 95 DEG C, adding acetone into the flask, and heating the flask to 150 DEG C; adding formaldehyde and benzaldehyde into the flask, maintaining the temperature at 150 DEG C, and performing reflux for 4h while performing stirring to obtain Mannich base; and compounding, by weight, 50-70% of Mannich base, 10-20% of phosphate, 10-20% of isopropanol, and 10-20% of sodium molybdate to prepare the corrosion inhibitor. The corrosion inhibitor can be used at a high temperature of 180 DEG C. When the concentration of the corrosion inhibitor is 100 ppm, an N80 iron sheet is corroded by stratum water of the Tahe Oilfield for 6h at 180 DEG C, and the corrosion inhibition rate is 90.04%.
Owner:CHINA PETROLEUM & CHEM CORP
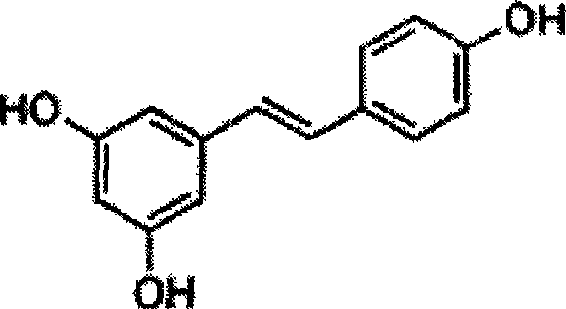
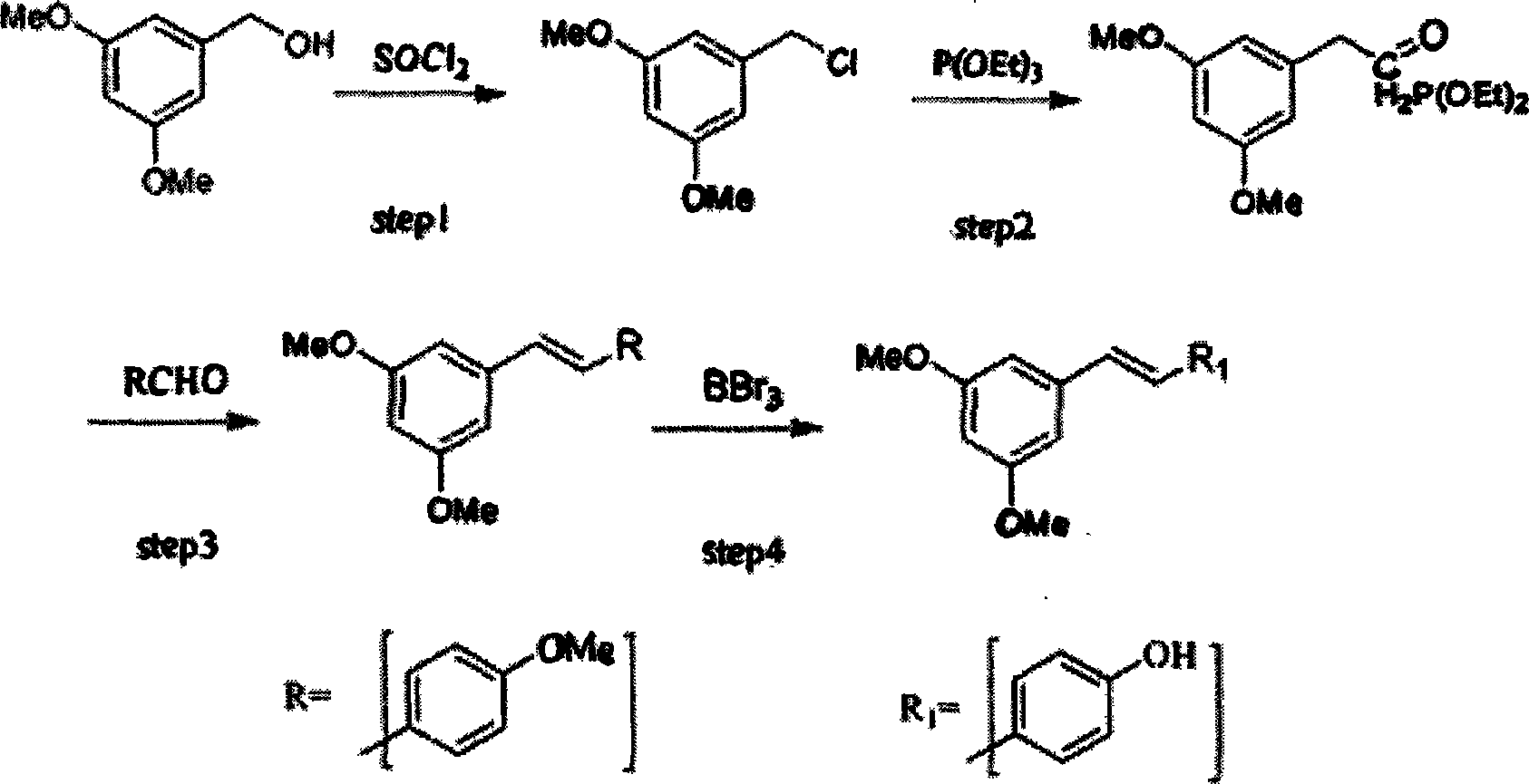
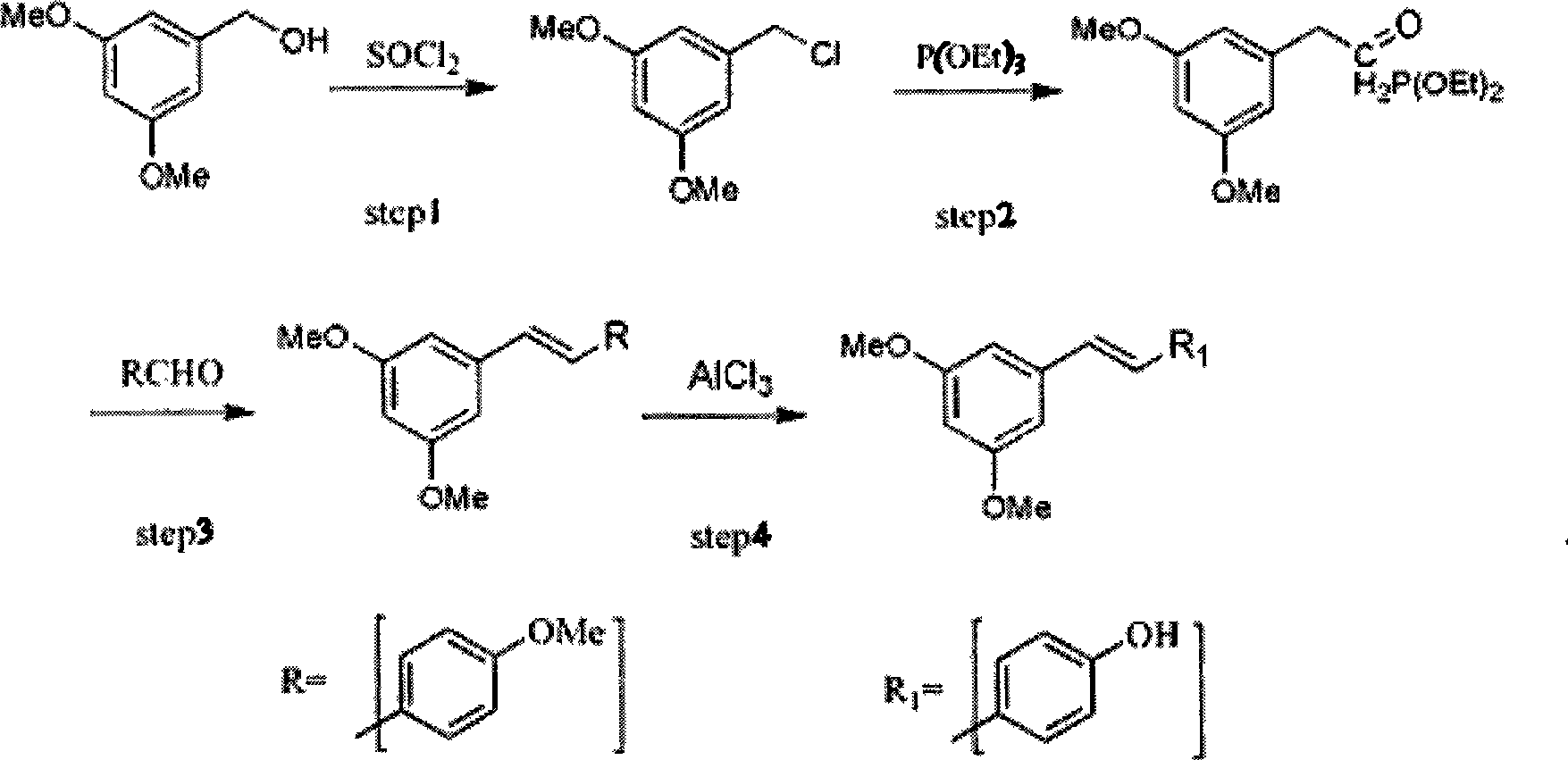
Method for synthesizing trans-resveratrol
InactiveCN101475451ALow priceHigh yieldOrganic chemistryOrganic compound preparationFood additiveDiethyl phosphate
The invention relates to a method for synthesizing trans-resveratrol as a functional food additive, which is characterized in that 3,5-methoxybenzylchloride is obtained after 3,5-methoxybenzylalcohol is chloro-substituted in DMF-triethylamine mixed solvent; 3,5-methoxy benzyl diethyl phosphate is obtained after the 3,5-methoxybenzylchloride reacts with triethyl phosphite; 3,4',5-trimethoxy toluylene is obtained after reaction products and anisic aldehyde are subjected to Witting reaction; and the trans-resveratrol is generated by removing methoxyl under the catalysis of N-methylaniline and aluminium trichloride. The method has the advantages of economical and readily available raw materials, mild reaction conditions, easy product purification, high reaction yield, friendliness to environment, simple operation and the like, thereby adapting to industrial production.
Owner:NANJING NORMAL UNIVERSITY
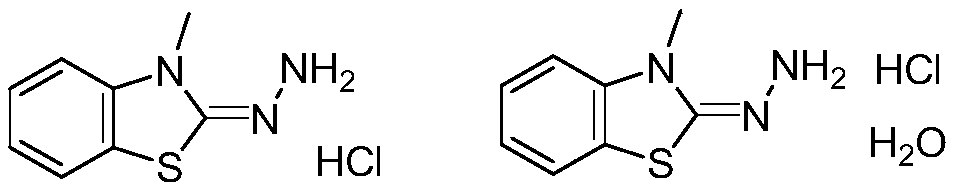
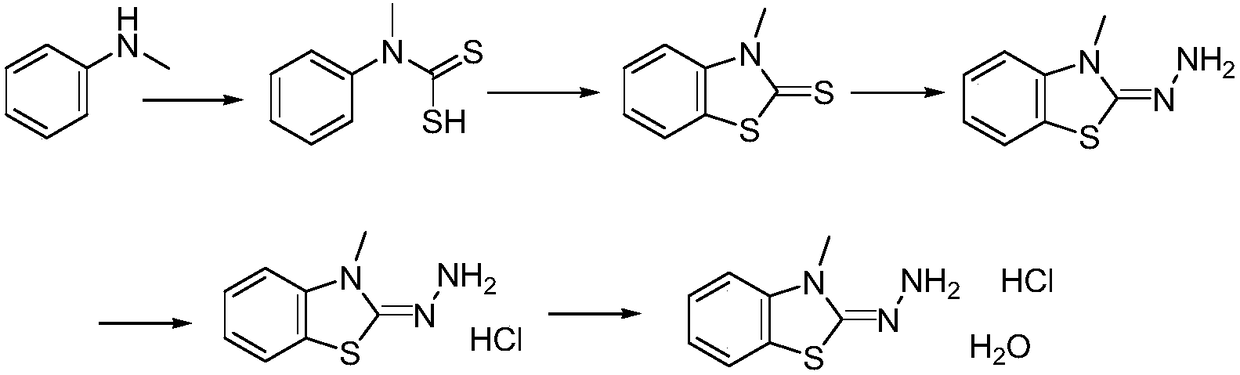
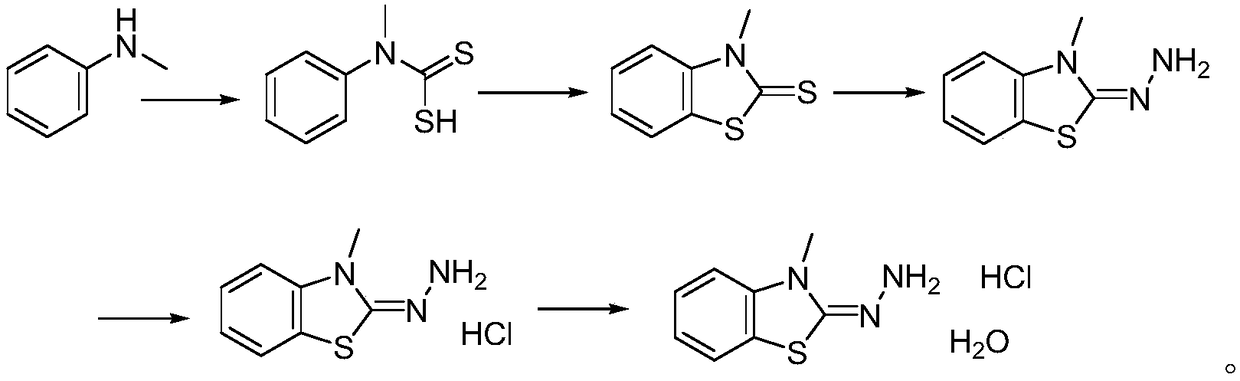
Method for synthesizing 3-methyl-2-benzothiazolinone hydrazone hydrochloride and its hydrate
ActiveCN109020918AShort synthetic routeEasy to operateOrganic chemistryOrganic synthesisHydrazine compound
The invention provides a method for synthesizing 3-methyl-2-benzothiazolinone hydrazone hydrochloride and its hydrate, and belongs to the field of organic synthesis. The method comprises the followingsteps: reacting N-methylaniline with carbon disulfide to obtain N-methyl-N-phenyldithiocarbamic acid, reacting the N-methyl-N-phenyldithiocarbamic acid with bromine to obtain 3-methylbenzothiazole-2-thione, reacting the 3-methylbenzothiazole-2-thione with hydrazine hydrate to generate 3-methyl-2-benzothiazolinone hydrazone, reacting the 3-methyl-2-benzothiazolinone hydrazone with hydrochloric acid to obtain 3-methyl-2-benzothiazolinone hydrazone hydrochloride, and re-crystallizing the 3-methyl-2-benzothiazolinone hydrazone hydrochloride in water to obtain the 3-methyl-2-benzothiazolinone hydrazone hydrochloride hydrate. The method has the advantages of cheap and easily available raw materials, simple operation process and potential industrial amplified application prospect.
Owner:青岛贞开生物医药技术有限公司
Corrosion inhibitor and preparation method thereof
ActiveCN105154049ACorrosion controlDrilling compositionBorehole/well accessoriesN-MethylanilineSodium molybdate
The invention relates to a corrosion inhibitor and a preparation method thereof. The corrosion inhibitor comprises, by weight: 50-70% of Mannich base, 10-20% of phosphate, 10-20% of isopropanol, and 10-20% of sodium molybdate. The preparation method of the corrosion inhibitor comprises: adding N-methylaniline into a three-neck flask, heating the flask to 80 DEG C, and adjusting the pH value to 2 with concentrated hydrochloric acid; heating the flask to 95 DEG C, adding acetone into the flask, and heating the flask to 150 DEG C; adding formaldehyde and benzaldehyde into the flask, maintaining the temperature at 150 DEG C, and performing reflux for 4h while performing stirring to obtain Mannich base; and compounding, by weight, 50-70% of Mannich base, 10-20% of phosphate, 10-20% of isopropanol, and 10-20% of sodium molybdate to prepare the corrosion inhibitor. The corrosion inhibitor can be used at a high temperature of 180 DEG C. When the concentration of the corrosion inhibitor is 100 ppm, an N80 iron sheet is corroded by stratum water of the Tahe Oilfield for 6h at 180 DEG C, and the corrosion inhibition rate is 90.04%.
Owner:CHINA PETROLEUM & CHEM CORP
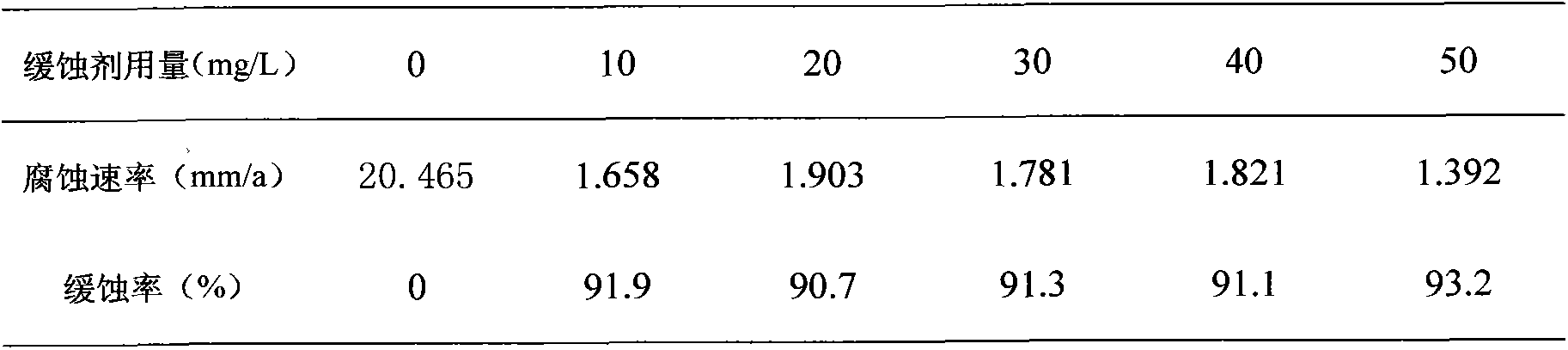
Method for preparing efficient acid-proof corrosion inhibiter
The invention discloses a method for preparing an efficient acid-proof corrosion inhibiter. The preparing method comprises the following specific steps: (1), adding ethanol, methanoic acid and N-methylaniline in a reactor, uniformly agitating, and then heating and raising the temperature to 70-90DEG C; (2), adding acetophenone into the reactor, simultaneously dropping in formaldehyde, and then, reacting for 3-5h; (3) adding benzyl chloride into the reactor and reacting for 1-3h; (4) stopping heating, naturally cooling to a room temperature, then adding propargyl alcohol into the reactor, and uniformly mixing to obtain the acid-proof corrosion inhibiter. The method for preparing the efficient acid-proof corrosion inhibiter has the advantages of mild reaction conditions and simple preparing technology. The acid-proof corrosion inhibiter prepared by the method has great corrosion resisting effect, is at a low cost, and has significant social and economic meanings after popularization and application.
Owner:GUANGDONG UNIV OF PETROCHEMICAL TECH
Preparation method of grading porous structure PNMA/lignosulfonic acid hybridized hydrogel
The invention discloses a preparation method of grading porous structure PNMA / lignosulfonic acid hybridized hydrogel. The preparation method comprises the following steps: I, dissolving sodium alginate, acidic dopant and N-methylaniline in deionized water to obtain a solution A; II, dissolving oxidant and lignosulfonate in the deionized water to obtain a solution B; III, uniformly mixing the solution A and the solution B, standing, and reacting; and IV, purifying and balancing in the deionized water, and finally filtering to obtain the purified PNMA / lignosulfonic acid hybridized hydrogel. Thepreparation method is simple, mild in reaction conditions, and low in requirement on the device performance; and the PNMA / lignosulfonic acid hybridized hydrogel can be obtained only by virtue of the supermolecule self-assembling behavior of a reaction system. The obtained hybridized hydrogel has a grading porous structure of a nano dimension, has higher specific capacity and long cycle life, is particularly applicable to an energy-storage electrode material, and is wide in application prospect in the fields such as sensing, catalysis and heavy metal ion adsorption.
Owner:东营悦来湖园区运营管理有限公司
Environment protectional antiknock additive for high-effective and clean gasoline for vehicle, and method for manufacturing same
InactiveCN1730626AStrong antiknockPromote environmental protectionLiquid carbonaceous fuelsIsobutanolN-Methylaniline
The gasoline additive provided by the invention comprises methanol, ethanol, butanol, isobutanol, N,N-dimethylaniline, and N-methylaniline. Its preparing process consists of mixing the components proportionally with a metering valve, loading into sealed storage tank, stewing 10-20 minutes, finally mixing homogeneously.
Owner:XIAN JIANNENG TECH
Multifunctional alcohol-based fuel
InactiveCN106244257AIncrease cetane numberImprove ignition characteristicsLiquid carbonaceous fuelsN-MethylanilineIsoamyl alcohol
The invention belongs to the technical field of fuels, and particularly relates to a multifunctional alcohol-based fuel. The multifunctional alcohol-based fuel is prepared from the following raw materials in parts by weight: 68 to 92 parts of methyl alcohol, 16 to 34 parts of diesel oil, 0.7 to 1.9 parts of amyl nitrate, 0.2 to 1.4 parts of poly-methoxyl dimethyl ether, 0.02 to 0.14 part of 5-amino-o-cresol, 0.3 to 1.5 parts of N-methylaniline, 0.1 to 1.3 parts of ferricinium, 0.01 to 0.07 part of tetrahydrocyclopentadiene, 0.03 to 0.15 part of isoamyl alcohol and 0.1 to 1.3 parts of a clearing agent. The multifunctional alcohol-based fuel disclosed by the invention has various functions of cleaning, oxidization prevention, combustion supporting, corrosion resisting, freezing resisting, smoke elimination and increase of a cetane number.
Owner:GUANGXI DONGQI ENERGY TECH CO LTD
Preparation method of catalyst for synthesis of N-methylaniline and application
ActiveCN107115881ASteps to Avoid Hydroreductive PretreatmentImprove catalytic performanceOrganic compound preparationAmino compound preparationN-MethylanilineSpinel
The invention belongs to the field of chemical processes, and in particular relates to a preparation method of a catalyst for synthesis of N-methylaniline and application. The catalyst is a copper-zinc-chromium-aluminum spinel catalyst, the catalyst does not need hydrogenation reduction pretreatment before being used, the single pass conversion rate of aniline is as high as 90% or more, and the selectivity of the N-methylaniline is as high as 98% or more.
Owner:CHANGZHOU UNIV
Synthesis method of hellebore aldehyde
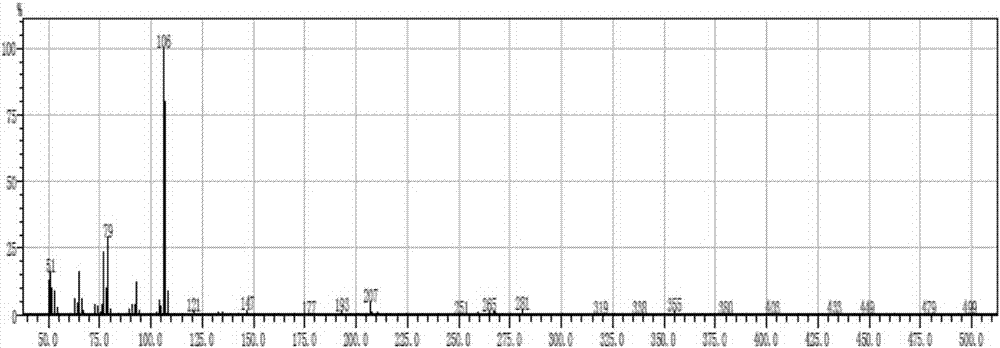
InactiveCN101735029BLow costReduce industrial wastewaterCarbonyl compound preparation by condensationN-MethylanilineFormylation reaction
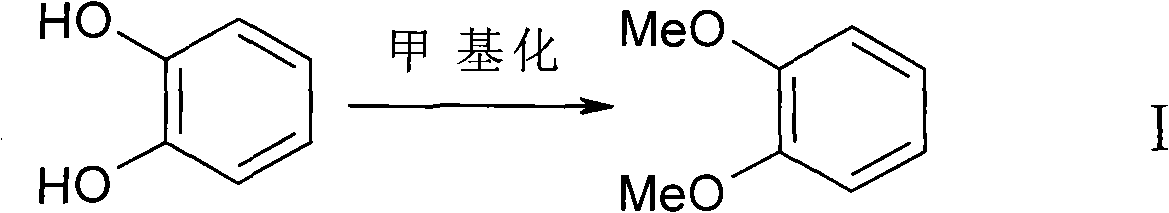
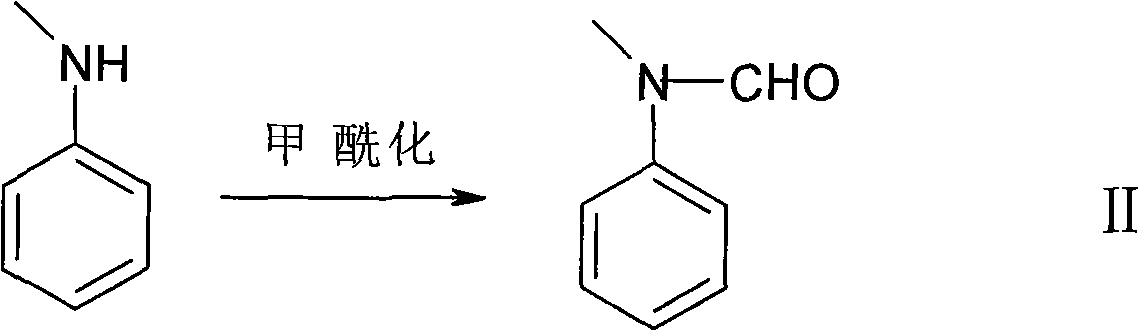
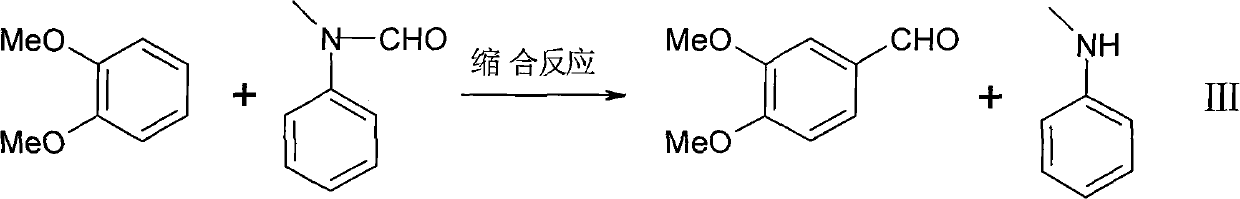
The invention discloses a hellebore aldehyde synthetic method. The method comprises the following steps of: synthesizing veratrole through methylation reaction by using pyrocatechol as a material; and synthesizing N-methylformanilide through formylation reaction using N-methylaniline as a material; reacting veratrole with N-methylformanilide at 20-100 DEG C for 8-24 h in the presence of a condensing agent and a catalyst to prepare the product with the purity of more than 97%. The invention is a clean and efficient synthesis method, the cost of the materials used in the invention is low, the formylation reagent can be recycled, and the industrial wastewater is less.
Owner:JIAXING EPOCHEM PHARMTECH
Preparation method of 2-methyl-7-azaindole
InactiveCN102827162ARaw materials are easy to getMild reaction conditionsOrganic chemistryN-MethylanilineAcetic anhydride
The invention relates to a preparation method of 2-methyl-7-azaindole. The method comprises the steps that: (1) 2-amino-3-methylpyridine is subjected to an acetic anhydride acylation reaction, such that 2-acetamido-3-methylpyridine is produced; and (2) 2-acetamido-3-methylpyridine is subjected to a cyclization reaction under the effects of sodium amide and N-methylaniline, such that 2-methyl-7-azaindole is produced. The method provided by the invention has the advantages of easy-to-obtain raw materials, relatively mild reaction conditions, easy control, high yield (wherein a total yield is higher than 60%), and low cost. The purity of the product 2-methyl-7-azaindole is high (wherein the purity is no lower than 99.5%). The product is suitable for industrialized productions.
Owner:ABA CHEM SHANGHAI
Preparation method of substituted indole C3 alkylation derivative
The invention relates to a preparation method of a substituted indole C3 alkylation derivative, and belongs to the technical field of organic synthesis. The preparation method of the substituted indole C3 alkylation derivative comprises the following steps: performing normal-temperature stirring reaction on substituted indole and a substituted N-methylaniline compound serving as raw materials in asolvent under the action of an organic dye photosensitizer and under the irradiation of an LED white light lamp, performing TLC monitoring until the reactions ends, and separating and purifying the reaction liquid to prepare the substituted indole C3 alkylation derivative. By adoption of the above technology, the invention provides a new method for synthesizing an indole C3 alkylation derivativeby taking cheap and easily available organic dye as a catalyst and under the induction of the visible light. According to the method, the reaction condition is mild, the catalyst is cheap and easily available, the yield is high, and the acid catalysis or transition metal catalysis synthesis method commonly used for indole alkylation is replaced.
Owner:ZHEJIANG UNIV OF TECH +1
Preparation method of rubber vulcanization accelerator-dimethyl-diphenyl thiuram disulfide
The invention provides a preparation method of a rubber vulcanization accelerator-dimethyl-diphenyl thiuram disulfide. In the preparation method, N-methylaniline, ammonia water with the concentration of 10-18% and carbon disulfide are taken as reactants for reacting in the presence of a catalyst-copper acetate so as to generate the dimethyl-diphenyl thiuram disulfide under the action of an oxidant, wherein, the mole ratio of the N-methylaniline to the ammonia water with the concentration of 15% to the carbon disulfide is 1: (1.05-1.3): (1.1-1.5), the catalyst accounts for 0.03-0.08% of the total mass of the N-methylaniline, and the oxidant is a mixed solution of hydrogen peroxide and sulfuric acid. The preparation method provided by the invention has the advantages that the hydrogen peroxide is taken as the oxidant, and less waste water is generated so that the preparation process is more environmentally-friendly, and in addition, a reaction can be performed at normal pressure so as to save energy; the ammonia water is taken as a solvent so as to prevent generation of waste mirabilite; the copper acetate is taken as the catalyst so as to shorten condensation reaction time; and the process flow is short, the production cost is low, and the product competitiveness can be enhanced.
Owner:鹤壁联昊新材料有限公司
Preparation method of N-methyl formyl aniline
InactiveCN101475501AHigh yieldReduce the amount of feedOrganic chemistryOrganic compound preparationN-MethylanilineSolvent
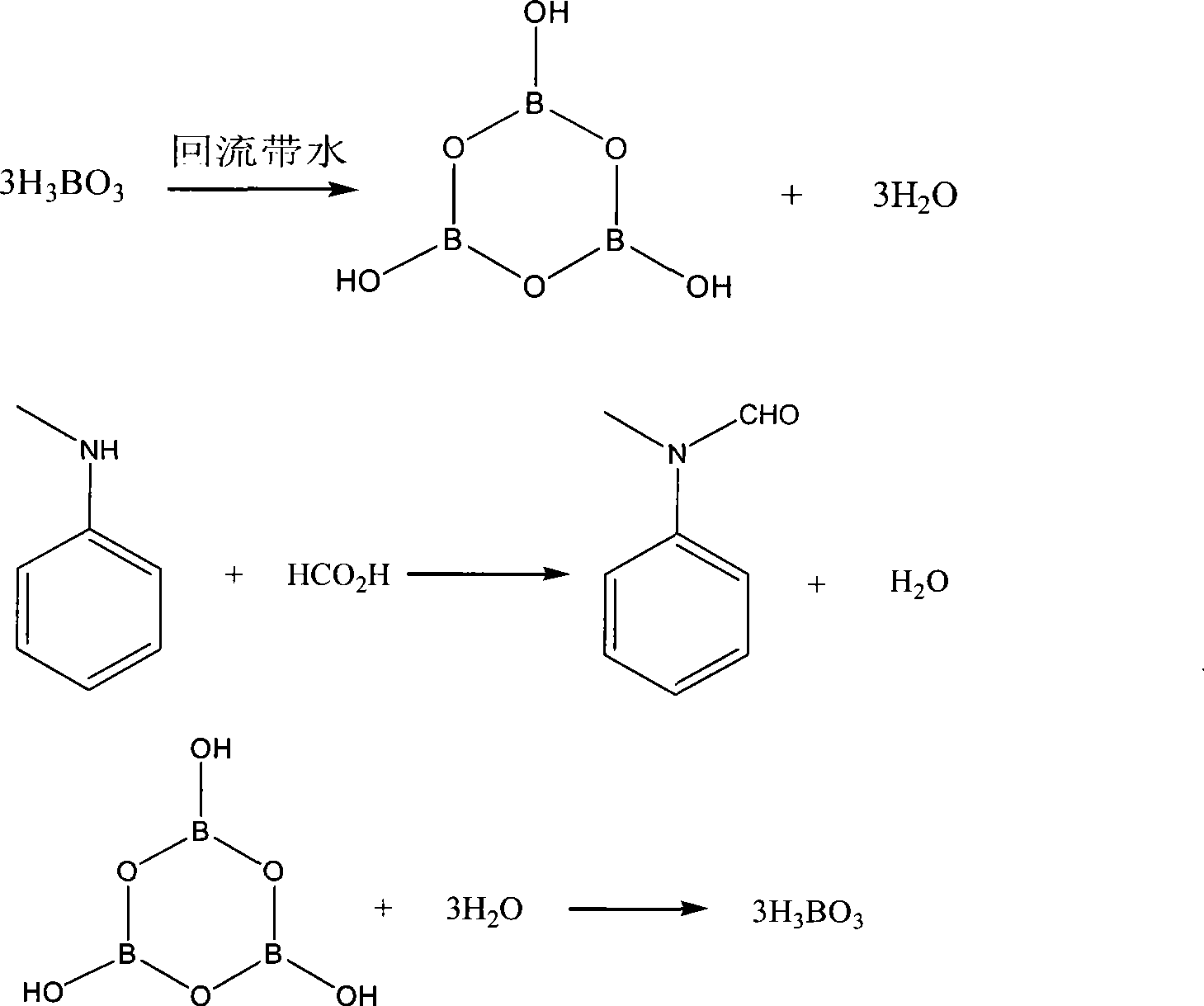
The invention discloses a method for preparing N-methyl formanilide. The prior method adopts N-methylaniline to react with formic acid to prepare the N-methyl formanilide, is balanced reaction of esterification and hydrolysis, and requires continuous removal of water generated during the reaction so as to require superfluous anhydrous formic acid and be difficult to react completely. The method comprises the following steps: firstly, performing refluxing and water-carrying on boric acid in the presence of a solvent which is dissolved in water, and reducing the temperature of the obtained anhydride metaborate which is not subjected to separation to be between 60 and 90 DEG C first; and secondly, directly adding the formic acid and the N-methylaniline into a solution for reaction, and obtaining the N-methyl formanilide. The method adopts the anhydride metaborate as a chemical dewatering agent to remove the water generated during the reaction, strikes the balance, makes the reaction quick and complete, does not require refluxing and water-carrying during synthesis of the N-methyl formanilide, and greatly reduces the feed capacity of the formic acid.
Owner:GAOYOU CITY ORGANIC CHEM FACOTRY +1
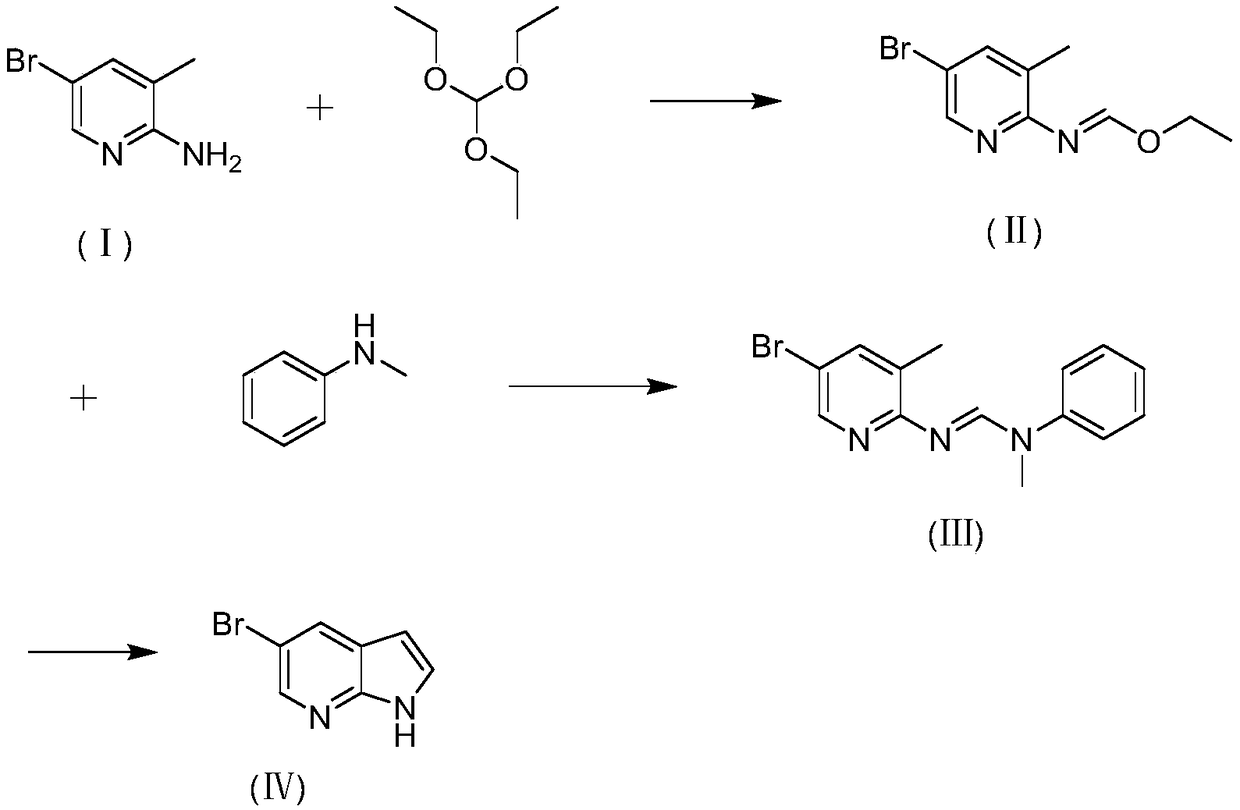
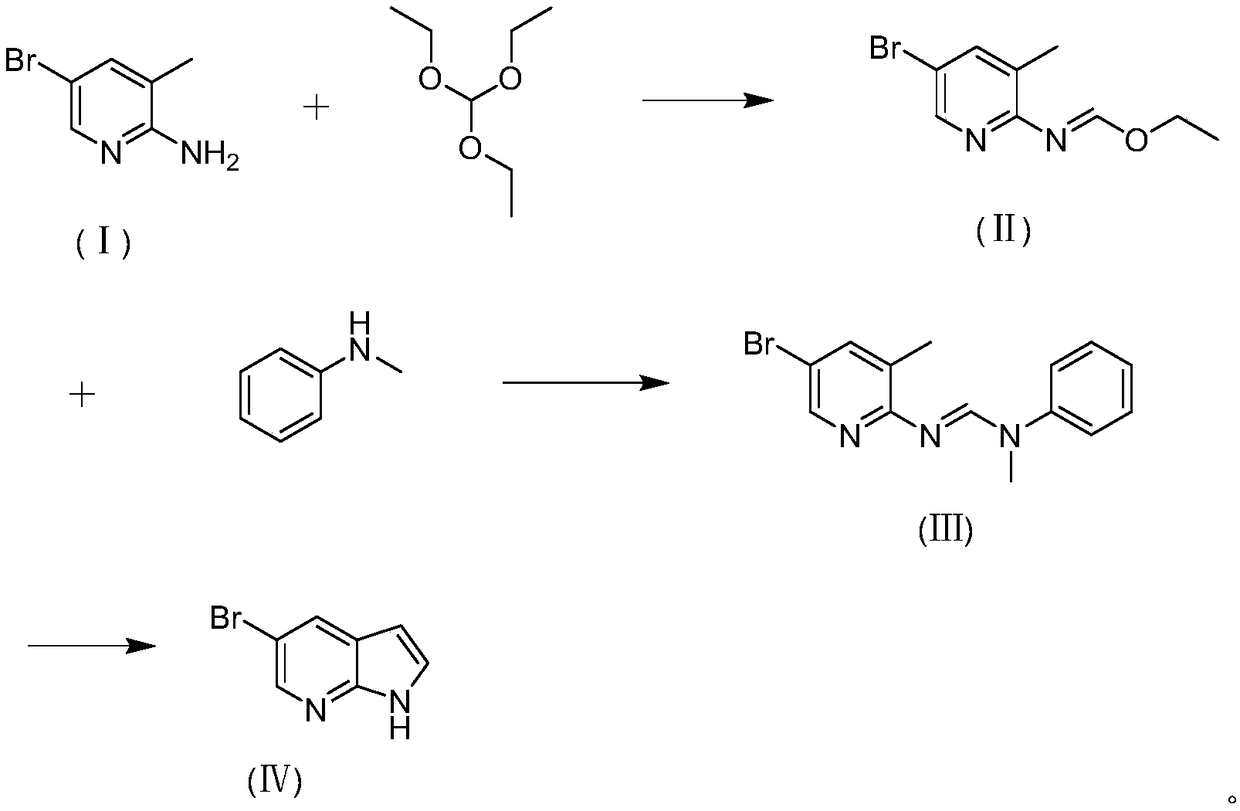
Preparation method of 5-bromine-7-azaindole
InactiveCN108752341AImprove reaction efficiencyHigh yieldOrganic chemistryN-MethylanilineBenzamidine
The invention discloses a preparation method of 5-bromine-7-azaindole. The method includes the steps that a, 2-amino-3-methyl-5-bromopyridine and triethyl orthoformate are utilized for obtaining N-(3-methyl-5-bromopyridine-2-radical) ethyl imidoformate; b, N-(3-methyl-5-bromopyridine-2-radical) ethyl ethyl imidoformate and N-methylaniline react to obtain N-(3-methyl-5-bromopyridine-2-radical)-N'-methyl-N'-benzamidine; c, N-(3-methyl-5-bromopyridine-2-radical)-N'-methyl-N'-benzamidine is subjected to a ring closing reaction under the effect of alkali, so that 5-bromine-7-azaindole is obtained.By synthesizing the imino acid ester compound, the one-step nucleophilic reaction is performed, then ring closing is performed, and finally 5-bromine-7-azaindole is obtained; synthesizing can be completed through three steps. The preparation method is easy to operate and suitable for industrialized production.
Owner:NANJING JIEYUN PHARMA TECH CO LTD
Method for synthesizing thiazine ketone
Owner:JIANGSU ANPON ELECTROCHEM
Method for preparing isothiocyano methyl formate
ActiveCN105481740ALess side effectsInhibit side effectsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDimethylaniline N-oxideSodium thiocyanate
The invention discloses a method for preparing isothiocyano methyl formate. The method is characterized in that a sodium thiocyanate aqueous solution and methylclhlorofonmate are taken as raw materials, a tubular reactor is adopted, and the isothiocyano methyl formate is synthesized continuously under the action of chloroethylene-supported N-alkyl-N-methylaniline serving as a solid catalyst. By adopting the method, the conventional kettle reaction method is changed. The tubular reactor is used for preparing the isothiocyano methyl formate by continuous synthesis, so that the reaction time is shortened greatly, reaction byproducts are reduced at the same time, side reactions of the isothiocyano methyl formate are avoided, and the reaction safety is enhanced. The chloroethylene-supported N-alkyl-N-methylaniline serving as the solid catalyst is adopted, so that the problem of odor pollution due to entrance of N,N-dimethylaniline serving as a catalyst into subsequent waste water in the conventional method is solved, and the environmental protection stress is lowered. Moreover, the catalyst can be used continuously for 300 hours, so that the production cost is lowered greatly. The method is low in equipment investment, is short in reaction period, is easy to operate, and is suitable for industrial production.
Owner:宁夏海利科技有限公司
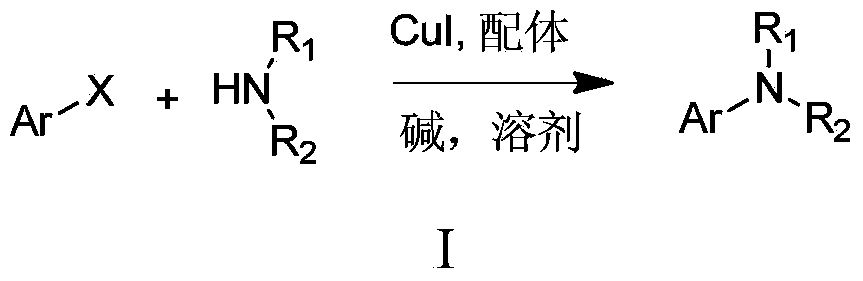
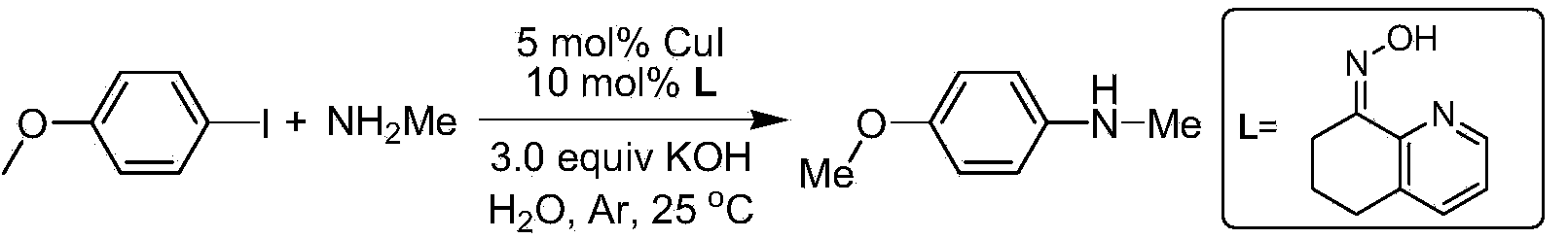
Method for N-arylation of alkane amine
InactiveCN103833561ALow pricePromotes N-arylation reactionCarboxylic acid nitrile preparationOrganic compound preparationN-MethylanilineChemical synthesis
The invention discloses a method for N-arylation of alkane amine, belonging to the technical field of chemical synthesis. The method comprises a step of making the alkane amine and an aromatic compound have an Ar-X reaction in the presence of a base and a solvent by taking a 2-pyridyl ketoxime compound as an additive and CuI as a catalyst so as to realize the N-arylation of the alkane amine. The method can be used for well promoting the proceeding of an N-arylation reaction, so that the N-arylation reaction between an alkane amine compound and an iodo-aromatic compound in a water liquid can be performed at room temperature, and the N-arylation reaction of a bromo-aromatic compound can be performed at the temperature of 65 DEG C; meanwhile, the 2-pyridyl ketoxime compound can be used for promoting lots of compounds containing various functional groups to have a Ullman coupling reaction, and particularly N-methylaniline and N'N-dimethylaniline compounds can be efficiently synthesized in the water liquid at room temperature, so the method disclosed by the invention has a quite wide substrate application range and a quite good application prospect.
Owner:HENGYANG NORMAL UNIV
Synthetic method for organic synthesis intermediate caproic acid
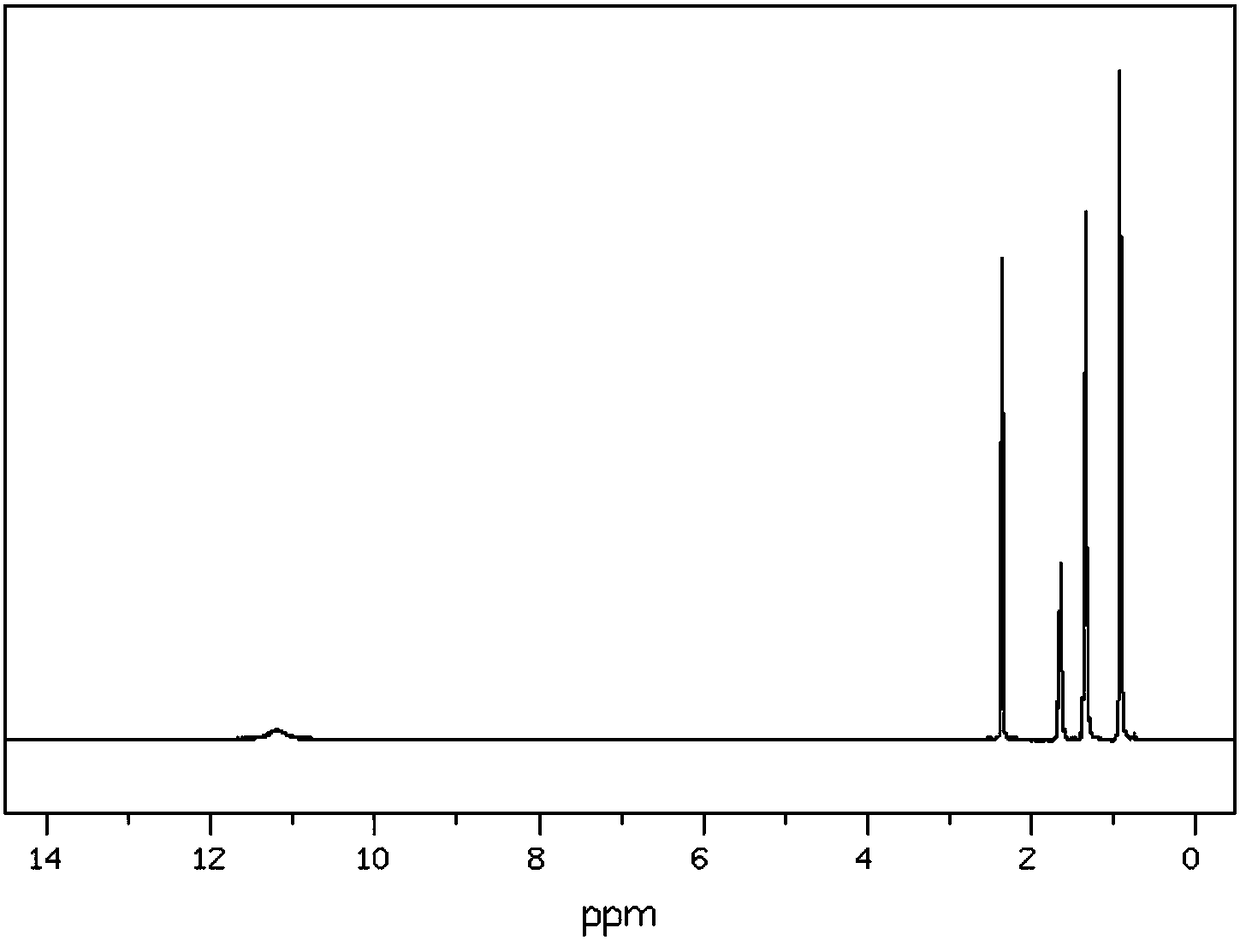
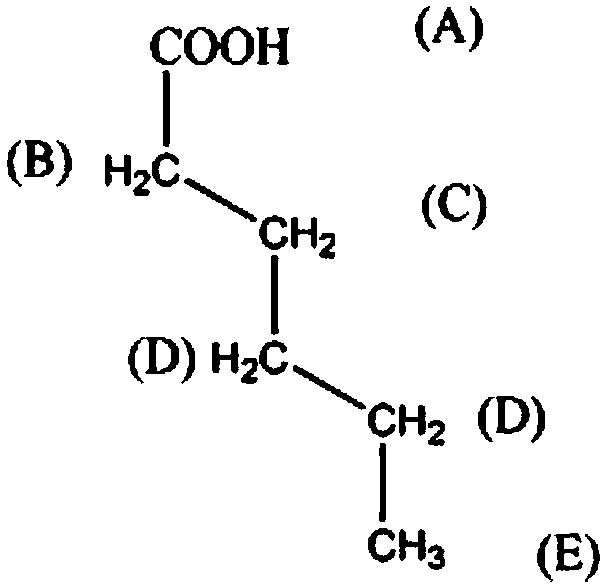
InactiveCN108238925AIncreased corrosion resistance requirementsHigh reaction yieldOrganic compound preparationCarboxylic compound separation/purificationN-MethylanilinePentaerythritol
The invention discloses a synthetic method for the organic synthesis intermediate caproic acid. The synthetic method comprises the following steps: adding 1-amino-2-bromo-heptane into a reaction vessel, controlling a stirring speed, adding a potassium chloride solution and raising a temperature; and adding praseodymia powder, raising the temperature, then adding a triacetin solution, continuing areaction, lowering the temperature, subjecting the obtained solution to layering, then carrying out washing with a sodium nitrate solution a plurality of times, carrying out washing with a 3-hexanonesolution a plurality of times, then carrying out washing with a N-methylaniline solution a plurality of times, carrying out recrystallization in a pentaerythritol solution, and then carrying out dehydration with a dehydrating agent to obtain the finished caproic acid.
Owner:CHENGDU QIANYE LONGHUA PETROLEUM ENG TECH CONSULTING
N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl formamidine preparation method
InactiveCN107235859ASimple processEasy to operateOrganic chemistryN-MethylanilineEthyl aminobenzoate
The invention discloses an N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl formamidine preparation method. According to the method, ethyl p-aminobenzoate, N-methylaniline and formic acid serve as starting raw materials to prepare N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl formamidine through one-step reaction. The method is simple in process and convenient to operate; a byproduct only comprises water, so that safety, environmental friendliness and freeness of pollution are realized.
Owner:DALIAN RES & DESIGN INST OF CHEM IND
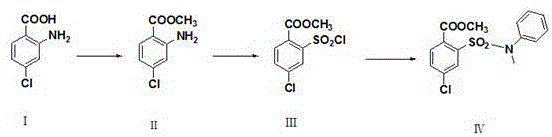
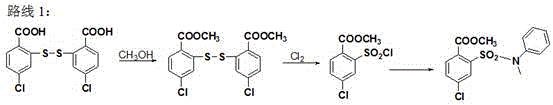
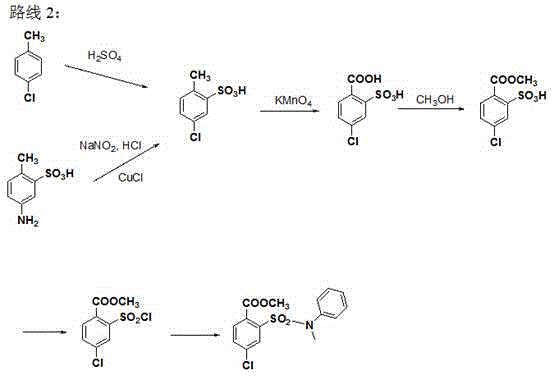
Synthetic method of 4-chloro-2-(N-methyl-N-phenyl sulfamoyl) methyl benzoate
ActiveCN105503668AEasy to operateShort stepsOrganic compound preparationSulfonic acid amide preparationN-MethylanilineSandmeyer reaction
The invention provides a synthetic method of 4-chloro-2-(N-methyl-N-phenyl sulfamoyl) methyl benzoate. The technical scheme is that 4-chloro-2-aminobenzoic acid is taken as a raw material and has an esterification reaction, a product is subjected to diazotization and the Sandmeyer reaction, methyl 4-chloro-2-(chlorosulfonyl)benzoate is prepared and subjected to condensation with N-methylaniline, and 4-chloro-2-(N-methyl-N-phenyl sulfamoyl) methyl benzoate is obtained. The synthetic method has the advantages of conciseness, high efficiency, mild conditions, no need of purification of an intermediate and simple operation, the total yield of three steps of reactions is as high as 65%, and the method is quite suitable for industrial production.
Owner:山东诚汇双达药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com