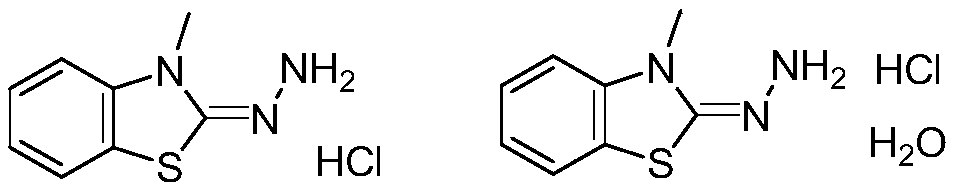
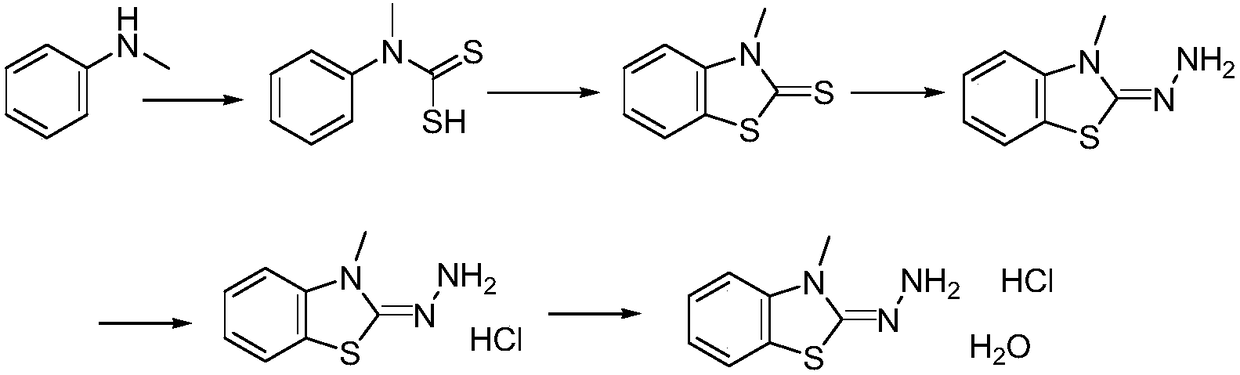
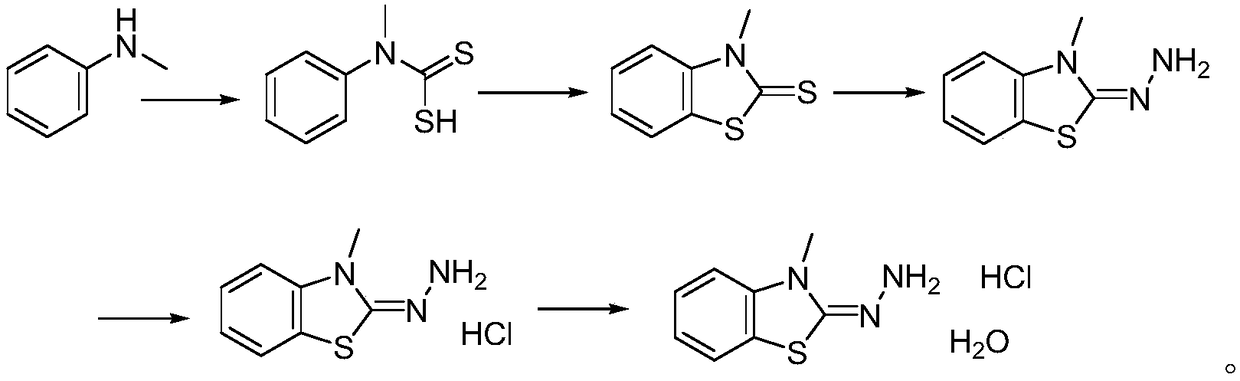
Method for synthesizing 3-methyl-2-benzothiazolinone hydrazone hydrochloride and its hydrate
A kind of technology of benzothiazolinone hydrazone hydrochloride and benzothiazolinone hydrazone, which is applied in the synthesis field of 3-methyl-2-benzothiazolinone hydrazone hydrochloride and its hydrate, and can solve the cost Expensive, troublesome operation and other problems, to achieve the effect of low cost, convenient operation and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) N-Methylaniline (0.1mol, 10.7g), triethylamine (0.11mol, 11g), carbon disulfide (0.1mol, 7.6g) were dissolved in 100mL of methanol, and stirred at 0°C for two hours, and then continue to react at room temperature for two hours. After the reaction is complete, the methanol solvent is spun off, 100mL of ethyl acetate is added, washed twice with water, dried over anhydrous sodium sulfate, and then the ethyl acetate is spun off and washed with methyl tert-butyl 15 g of N-methyl-N-phenyldithiocarbamate was obtained by crystallization of base ether, with a yield of 82%. HNMR (DMSO-d 6 ): δppm=7.29(d,2H),7.17(m,3H),3.66(s,3H).
[0027] (2) Dissolve the compound N-methyl-N-phenyldithiocarbamate (0.1mol, 18.3 grams) obtained in step (1) in 200mL of dichloromethane, add bromine (0.1 mol, 16 grams) for reaction, about half an hour dropwise, and then stirred for 2 hours, the reaction was completed, washed twice with water, the organic layer was dried with anhydrous sodium sul...
Embodiment 2
[0032] (1) N-methylaniline (0.1mol, 10.7g), triethylamine (0.12mol, 12g), carbon disulfide (0.1mol, 7.6g) were dissolved in 110mL of ethanol, and stirred at 0°C for two hour, and then continue to react for two hours at room temperature. After the reaction is completed, the ethanol solvent is spun off, 120 mL of ethyl acetate is added, washed twice with water, dried over anhydrous sodium sulfate, and then rotary evaporated to remove ethyl acetate to obtain a crude product. Then crystallize with methyl tert-butyl ether to obtain 15.2 g of N-methyl-N-phenyldithiocarbamate, with a yield of 83%.
[0033] (2) The compound N-methyl-N-phenyldithiocarbamate (0.1mol, 18.3 grams) obtained in step (1) was dissolved in 200 mL of chloroform, and bromine (0.11 mol, 17.6 g) to react, about half an hour to complete the dropwise addition, and then stir the reaction for 2 hours, the reaction was completed, washed twice with water, the organic layer was dried with anhydrous sodium sulfate, concen...
Embodiment 3
[0038] (1) N-methylaniline (0.1mol, 10.7g), triethylamine (0.13mol, 13g), carbon disulfide (0.11mol, 8.4g) were dissolved in 120mL of methanol, and stirred at 0°C for two hour, and then continue to react at room temperature for two hours. After the reaction is completed, the methanol solvent is spun off, and 120 mL of ethyl acetate is added to dissolve, then washed twice with water, dried over anhydrous sodium sulfate, and the ethyl acetate is spun off to obtain the crude product , crystallized with methyl tert-butyl ether to obtain 15.5 g of N-methyl-N-phenyldithiocarbamate, with a yield of 85%.
[0039] (2) The compound N-methyl-N-phenyldithiocarbamate (0.1mol, 18.3 grams) obtained in step (1) was dissolved in 220mL of dichloromethane, and bromine (0.12 mol, 19.2 grams) to react, about half an hour dropwise, and then stirred for 2 hours, the reaction was completed, first washed with sodium bicarbonate solution, then washed once with water, the organic layer was dried with an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com