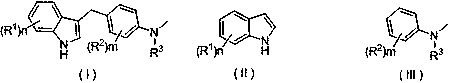
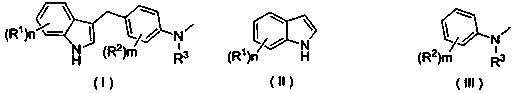Preparation method of substituted indole C3 alkylation derivative
A substituent and alkylation technology, applied in the field of organic synthesis, can solve the problems of high price and complex catalyst preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Derivative Ia (R 1 =R 2 = H, R 3 = methyl) synthesis
[0023] Weigh indole (0.5 mmol), N , N -Dimethylaniline (5 eq.) and 1.5 mol% Eosin B were placed in a 25 mL Schlenk reaction tube, then 4 mL of acetonitrile and 1 mL of water were added, and the reaction was carried out under a 15 W LED white light under the condition of air ventilation, at room temperature The reaction was stirred at lower temperature and monitored by TLC. After 24 h, the reaction was completed, the solvent was removed, and the concentrated solution was separated by column chromatography (petroleum ether / ethyl acetate=10: 1, V / V) to obtain a white solid, i.e. derivative Ia . Melting point 139-141 ° C, yield 71%.
[0024] of the compound 1 H NMR, 1 C NMR analysis data are described below,
[0025] 1 H NMR (CDCl 3 , 500 MHz) δ 7.94 (brs, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.35-7.37 (d, J = 8.0 Hz, 1H), 7.17-7.20 (m, 3H), 7.09 (t, J = 7.5 Hz, 1H), 6.91(s, 1H), 6.71 (d, J = 8.5 ...
Embodiment 2
[0026] Example 2 Derivatives Ib (R 1 = N -Methyl, R 2 = H, R 3 = methyl) synthesis
[0027] weigh N -Methylindole (0.5 mmol), N , N -Dimethylaniline (5.5 eq.) and 2.0 mol% Eosin Y-2Na were placed in a 25 mL Schlenk reaction tube, and then 5 mL of acetonitrile was added, and the reaction was placed under a 20 W LED white light under the condition of air ventilation, and the reaction was carried out at room temperature The reaction was stirred and followed and monitored by TLC. After 24 h, the reaction was completed, the solvent was removed, and the concentrated solution was separated by column chromatography (petroleum ether / ethyl acetate=20:1, V / V) to obtain a white solid, namely derivative Ib. Melting point 151-154 ° C, yield 70%.
[0028] of the compound 1 H NMR, 1 C NMR analysis data are described below,
[0029] 1 H NMR (CDCl 3 , 500 MHz) δ 7.62 (d, J = 8.0 Hz, 1H), 7.34 (d, J = 8.5 Hz,1H), 7.28 (m, 1H), 7.26-7.21 (m, 2H), 7.14 (t, J = 8.0 Hz, 1H), 6.80-6...
Embodiment 3
[0030] Example 3 Derivatives Ic (R 1 = N , 2-Dimethyl, R 2 = H, R 3 = methyl) synthesis
[0031] weigh N ,2-Dimethylindole (0.5 mmol), N , N -Dimethylaniline (6.0 eq.) and 2.2 mol% Eosin Y were placed in a 25 mL Schlenk reaction tube, then 5 mL of acetonitrile and 1 mL of water were added, and the reaction was carried out under a 15 W LED white light under the condition of air ventilation, Stir the reaction at room temperature, track and monitor with TLC, after 30 h, the reaction is complete, the solvent is removed, and the concentrated solution is separated by column chromatography (petroleum ether / ethyl acetate=10:1, V / V) to obtain a white solid, the derivative Ic. Melting point 126-128 ° C, yield 67%.
[0032] of the compound 1 H NMR, 1 C NMR analysis data are described below,
[0033] 1 H NMR (CDCl 3 , 500 MHz) δ 7.45 (dd, J = 8.0, 7.5 Hz, 2H), 7.27-7.20 (m,2H), 7.11 (t, J = 8.0 Hz, 2H), 6.98 (t, J = 7.5 Hz, 1H), 6.67 (d, J = 8.5 Hz,1H), 4.17 (s, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com