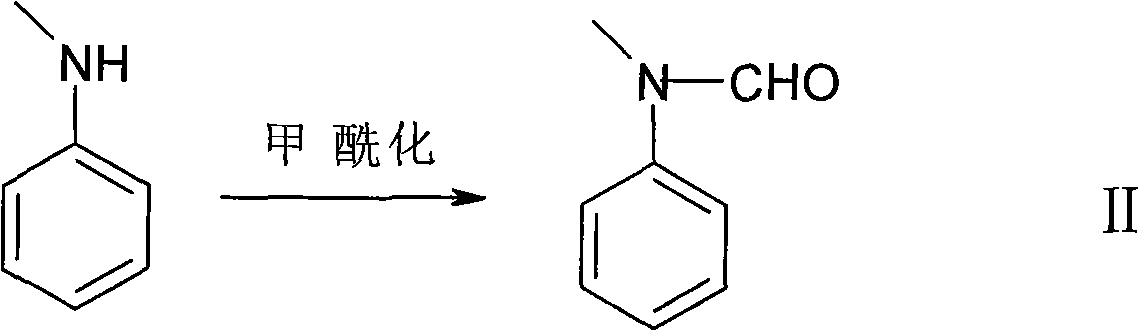
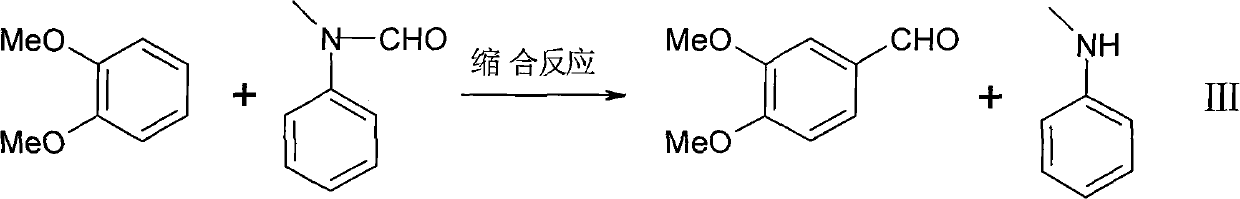
Synthesis method of hellebore aldehyde
A synthetic method, the technology of veratraldehyde, applied in the field of synthesizing veratraldehyde, can solve the problems of high pollution, low yield, limiting the use efficiency of reactors, etc., and achieve the effect of less industrial waste water and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
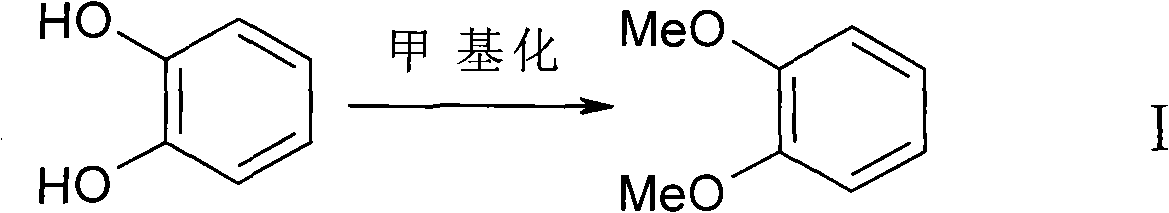
[0028] Methylation Reaction-Synthesis of Veratrole
[0029] 110 kilograms (1kmmol) of catechol and 120 kilograms of dimethyl sulfate are added to the reactor, and 300 kilograms of distilled water are added. Under stirring condition, add 184.8 kilograms (2.2kmol) sodium bicarbonate in batches, until completely no bubbles are produced. The reaction system was cooled to -5°C. Pass about 151.5 kg (3 kmol) of dichloromethane into the reaction system, and seal the reaction system. React under 80 degree steam bath for 12 hours. Cool to room temperature and open the reaction kettle. Separate the organic layer. The aqueous layer was extracted once with dichloroethane. The organic layers were combined and dried over anhydrous sodium sulfate. Dichloroethane was recovered by atmospheric distillation, and 125 kg of product was obtained by vacuum distillation (90% yield). The results of elemental analysis of veratrole showed: C, 69.54; H, 7.30; O, 23.16; H NMR spectrum data: δ(CDCl3)...
Embodiment 2
[0035] Methylation Reaction-Synthesis of Veratrole
[0036]110 kilograms (1kmmol) of catechol and 122 kilograms (1kmmol) of methyl iodide were added to the reactor, and 200 kilograms of distilled water and 100 kilograms of acetone were added. Under stirring condition, add 233.2 kilograms (2.2kmol) sodium carbonate in batches, produce completely without bubble. The reaction system was cooled to 0°C. About 151.5 kg (3 kmol) of dichloromethane was introduced into the reaction system. Closed reaction system. React for 12 hours in a steam bath. Cool to room temperature and open the reaction kettle. Separate the organic layer. The aqueous layer was extracted once with dichloroethane. The organic layers were combined and dried over anhydrous sodium sulfate. Dichloroethane was recovered by atmospheric distillation, and 110 kg of product was obtained by vacuum distillation (yield 80%). Elemental analysis data: C, 69.54; H, 7.30; O, 23.16; H NMR spectrum data: δ(CDCl3): 6.88(s, ...
Embodiment 3
[0042] Methylation Reaction-Synthesis of Veratrole
[0043] 110 kilograms (1kmmol) of catechol and 122 kilograms (1kmmol) of methyl iodide were added to the reactor, and 200 kilograms of distilled water and 100 kilograms of acetone were added. Under stirring condition, add 233.2 kilograms (2.2kmol) sodium carbonate in batches, produce completely without bubble. The reaction system was cooled to -3°C. About 151.5 kg (3 kmol) of dichloromethane was introduced into the reaction system. Closed reaction system. React for 12 hours under steam bath. Cool to room temperature and open the reaction kettle. Separate the organic layer. The aqueous layer was extracted once with dichloroethane. The organic layers were combined and dried over anhydrous sodium sulfate. Dichloroethane was recovered by atmospheric distillation, and 110 kg of product was obtained by vacuum distillation (yield 80%). Elemental analysis data: C, 69.54; H, 7.30; O, 23.16. H NMR spectrum data: δ(CDCl3): 6.88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com