Preparation method of N-methyl formyl aniline
A technology of methylformanilide and methylaniline, which is applied in the field of synthesis of organic intermediates, can solve the problems of high cost, high pollution or toxicity, and three wastes, and achieve the effect of reducing the amount of feed and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
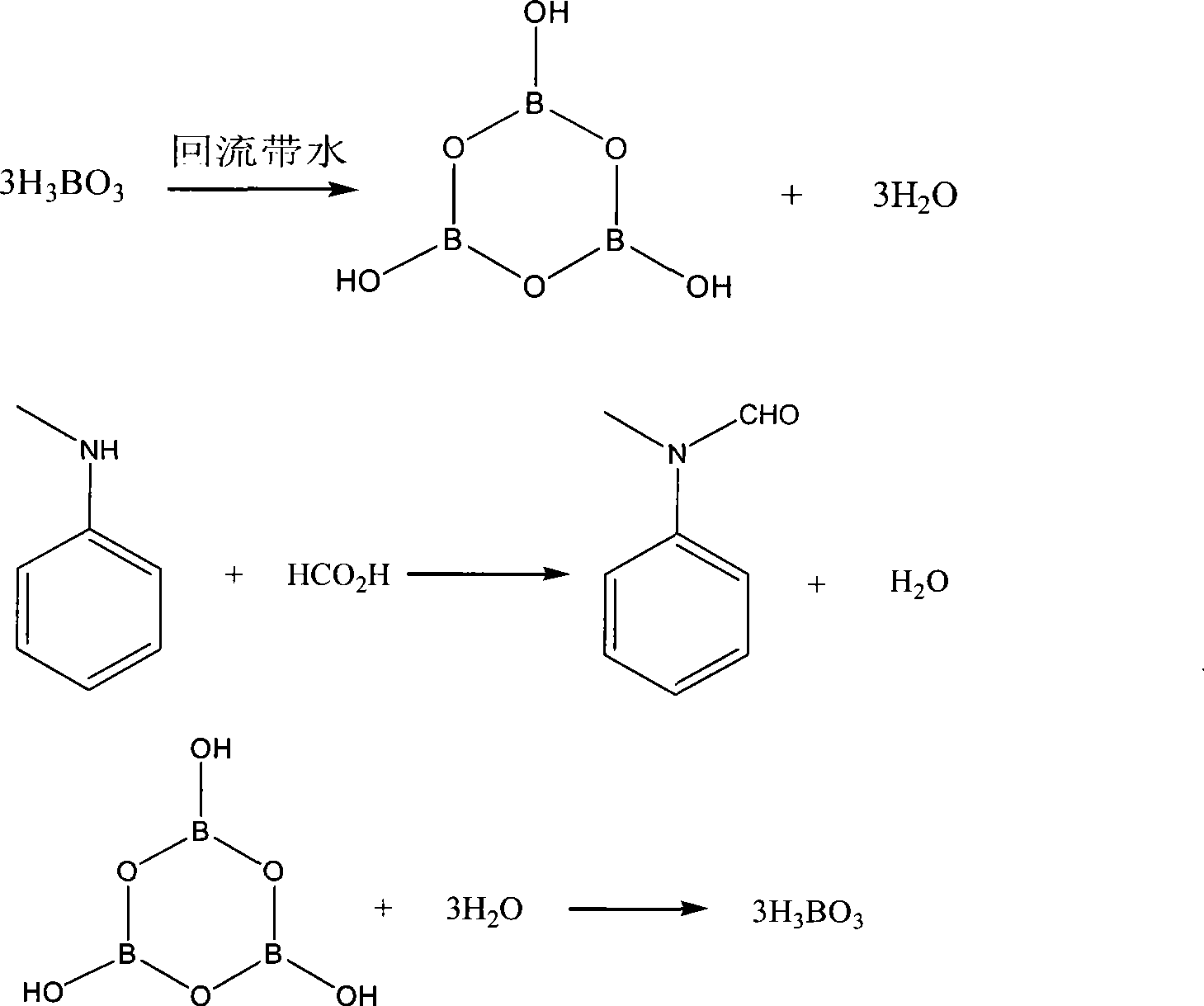
[0014] Embodiment 1: the preparation of N-methyl formanilide
[0015] In a 500ml three-necked flask, add boric acid 84g (1.4mol), toluene 200ml, reflux with water, take out 23.5 grams of water (1.3mol) after about 2 hours, cool to 90°C, add 40 grams of formic acid with a content of 88% ( Contain 4.8 grams of water and 0.78mol formic acid) and N-methylaniline 70 grams (0.65mol), insulated under mechanical stirring 90-100 ℃ of reaction 4.5 hours, gas chromatographic follow-up analysis shows that conversion rate is greater than 99%, drops to room temperature, Filter, wash the filter residue with 50ml of toluene, and dry to obtain 82 grams of white solid boric acid, which can be used mechanically. After the filtrates were combined, they were washed with 50 ml of saturated saline, separated into layers, and then the solvent was recovered under reduced pressure to dryness, and the fraction at 80-90 °C / 7mmHg was collected to obtain 81.5 grams of the product, which was a light yellow ...
Embodiment 2
[0021] Embodiment 2: the preparation of 3,4-dimethoxybenzaldehyde
[0022] In a 500ml three-necked flask, add 34.5g (0.25mol) of o-phthalamide and 34.5g (0.225mol) of phosphorus oxychloride, heat to 85°C, and drop into the mixture prepared in Example 1 within 1 hour under stirring. 27 g (0.2 mol) of N-methylformanilide. Insulate and react at 80-90°C for 6 hours, follow the progress of the reaction with TLC, developer ethyl acetate:petroleum ether=1:1. After reacting for 6 hours, the thin layer tracking raw material basically disappeared (developer: ethyl acetate: petroleum ether = 1:3), add 100ml of toluene to dilute, cool down to room temperature, add 100ml of water, stir for 1 hour, and separate layers. After the organic layer was dried over anhydrous sodium sulfate, the solvent was recovered, the residue was rectified by an oil pump, and the 104-108 ° C / 1mmHg fraction was collected to obtain 27.6 g (0.166 mol, gas phase content 98.5%) of the product 3,4-dimethoxybenzaldehy...
Embodiment 3
[0028] Embodiment 3: the preparation of 2,4-dimethoxybenzaldehyde
[0029] In a 500ml three-necked flask, add m-xylylene dimethyl ether 34.5g (0.25mol) and phosphorus oxychloride 34.5g (0.225mol), heat to 85°C, and drop into the mixture prepared in Example 1 within 1 hour under stirring. 27 g (0.2 mol) of N-methylformanilide. Insulate and react at 80-90°C for 3 hours, track the progress of the reaction with TLC, developer ethyl acetate:petroleum ether=1:1. After reacting for 3 hours, the TLC raw material basically disappeared (developer: ethyl acetate:petroleum ether=1:3), add 100ml of toluene to dilute, cool down to room temperature, add 100ml of water and stir for 1 hour, and separate layers. After the organic layer was dried over anhydrous sodium sulfate, the solvent was recovered, the residue was rectified by an oil pump, and the 109-114 ° C / 1mmHg fraction was collected to obtain 25.5 g (0.154 mol, gas phase content 98.2%) of the product 2,4-dimethoxybenzaldehyde. Yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com