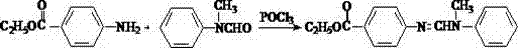N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl formamidine preparation method
An ethoxycarbonylphenyl, phenylformamidine technology, applied in the field of fine chemicals, can solve the problems of increased cost, high atom utilization, waste of resources, etc., and achieves mild reaction conditions, high atom utilization, and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 165g (1mol) of ethyl p-aminobenzoate, 107g (1mol) of N-methylaniline, 2g of p-toluenesulfonic acid, and 200mL of toluene with water agent into a 1000mL reaction flask, stir and heat to 50-60℃, within 2h 54.12g (1mol) of formic acid (content 85%) was added dropwise, the temperature was raised to reflux, and the generated water was continuously separated. After reaction for about 5 hours, the water was stopped when there was no more water.
[0022] The reaction liquid was distilled at atmospheric pressure to recover the water-carrying toluene. After the toluene was recovered, the remaining reaction liquid was distilled under reduced pressure to obtain the light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N '-Phenyl formamidine 251g, yield 89%, purity 99.5%.
Embodiment 2
[0024] Add 165g (1mol) of ethyl p-aminobenzoate, 117.7g (1.1mol) of N-methylaniline, 10g of p-toluenesulfonic acid, and 200mL of toluene with water into a 1000mL reaction flask, stir and heat to 50-60℃, Add 59.53g (1.1mol) formic acid (content 85%) dropwise within 2h, heat up to reflux reaction, and continuously separate the generated water. After reacting for about 5h, stop water separation when there is no more water out.
[0025] The reaction liquid was distilled at atmospheric pressure to recover the water-carrying toluene. After the toluene was recovered, the remaining reaction liquid was distilled under reduced pressure to obtain the light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N '-Phenyl formamidine 262g, yield 93%, purity 99.4%.
Embodiment 3
[0027] Add 165g (1mol) of ethyl p-aminobenzoate, 128.4g (1.2mol) of N-methylaniline, 5g of p-toluenesulfonic acid, and 200mL of toluene with water into a 1000mL reaction flask, stir and heat to 50-60℃, Add 81.17g (1.5mol) formic acid (content 85%) dropwise within 2h, heat up to reflux reaction, and continuously separate the generated water. After reacting for about 5h, stop the water separation when there is no more water out.
[0028] The reaction liquid was distilled at atmospheric pressure to recover the water-carrying toluene. After the toluene was recovered, the remaining reaction liquid was distilled under reduced pressure to obtain the light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N '-Phenyl formamidine 260g, yield 92%, purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com