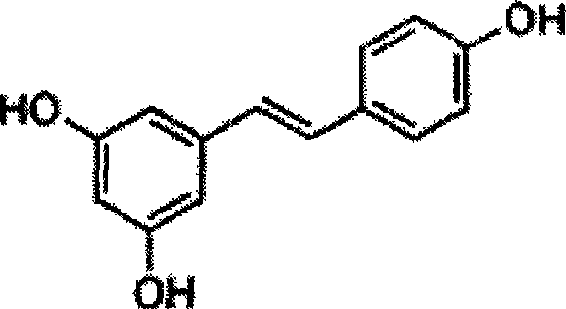
Method for synthesizing trans-resveratrol
A technology of resveratrol and reaction time, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high price, poor stability, and large dosage of boron tribromide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
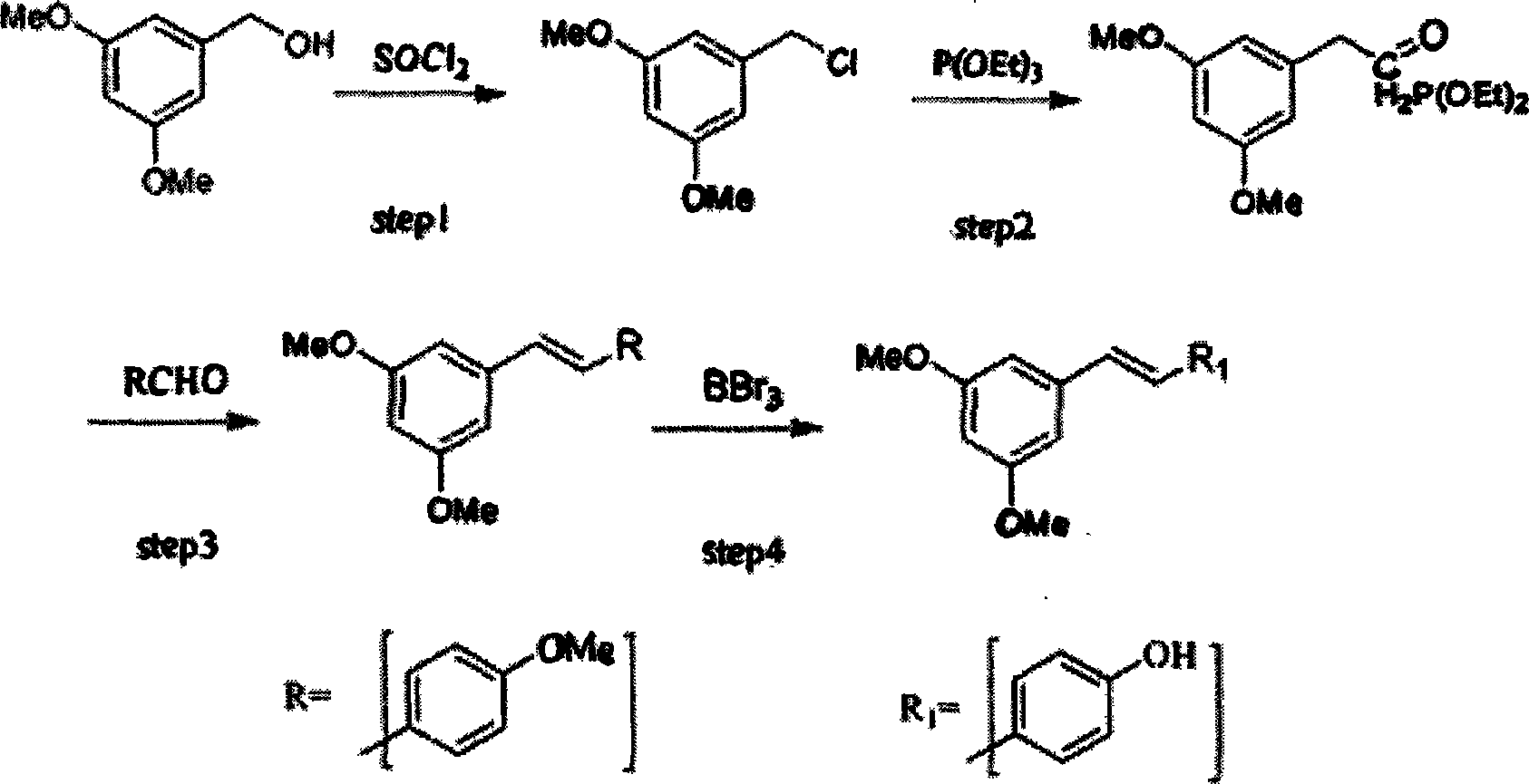
[0026] Step 1): In a 250ml three-necked flask, 30g of 3,5-methoxybenzyl alcohol was dissolved in a mixed solution of 100ml of DMF and 10ml of triethylamine, and 27ml of thionyl chloride was added dropwise in a water bath at 20°C using a constant pressure dropping funnel (The molar ratio of 3,5-methoxybenzyl alcohol to thionyl chloride is 1:2), after 1 hour of dropwise addition, keep stirring for 4 hours, pour the solution into water at 0°C, precipitate solid, and filter out the solid , washed the solid with water until neutral, and dried it under vacuum at 50° C. for 2 hours to obtain 32.2 g of white crystals, with a yield of 96.7%.
[0027] Step 2): A 250ml three-necked flask was connected to a reflux condenser, and 32.2g of the unpurified product of step 1) and 120ml of triethyl phosphite were added, stirred and reacted in an oil bath at 120°C for 5 hours, then vacuum distilled at 80°C with a vacuum of 5torr , until no liquid was distilled off to obtain 48.3 g of red oily li...
Embodiment 2
[0033] Step 1): In a 250ml three-necked flask, 35g of 3,5-methoxybenzyl alcohol was dissolved in a mixed solution of 100ml of DMF and 5ml of triethylamine, and 23.5ml of thionyl chloride was added dropwise using a constant pressure dropping funnel in a 20°C water bath (The molar ratio of 3,5-methoxybenzyl alcohol to thionyl chloride is 1:1.5), after 1 hour of dropwise addition, keep stirring for 3 hours, pour the solution into 0°C water, precipitate solid, and filter out the solid , washed the solid with water until neutral, and dried it under vacuum at 50° C. for 2 hours to obtain 36.6 g of white crystals, with a yield of 94.2%.
[0034] Step 2) 3) 4) is basically the same as embodiment 1, the difference is
[0035] In step 4), N-methylformylaniline and aluminum trichloride were used as catalysts and reacted at 120° C. for 4 hours; 50 ml of ethanol: water = 1: 1.5 mixed solvent was used for recrystallization.
Embodiment 3
[0037] Step 1): In a 250ml three-necked flask, dissolve 26g of 3,5-methoxybenzyl alcohol in a mixed solution of 100ml of DMF and 30ml of triethylamine, add 35ml of thionyl chloride dropwise in a water bath at 20°C using a constant pressure dropping funnel (The molar ratio of 3,5-methoxybenzyl alcohol to thionyl chloride is 1:3), after 1 hour of dropwise addition, keep stirring for 5 hours, pour the solution into water at 0°C, precipitate solid, and filter out the solid , washed the solid with water until neutral, and dried it under vacuum at 50° C. for 2 hours to obtain 27.5 g of white crystals, with a yield of 95.4%.
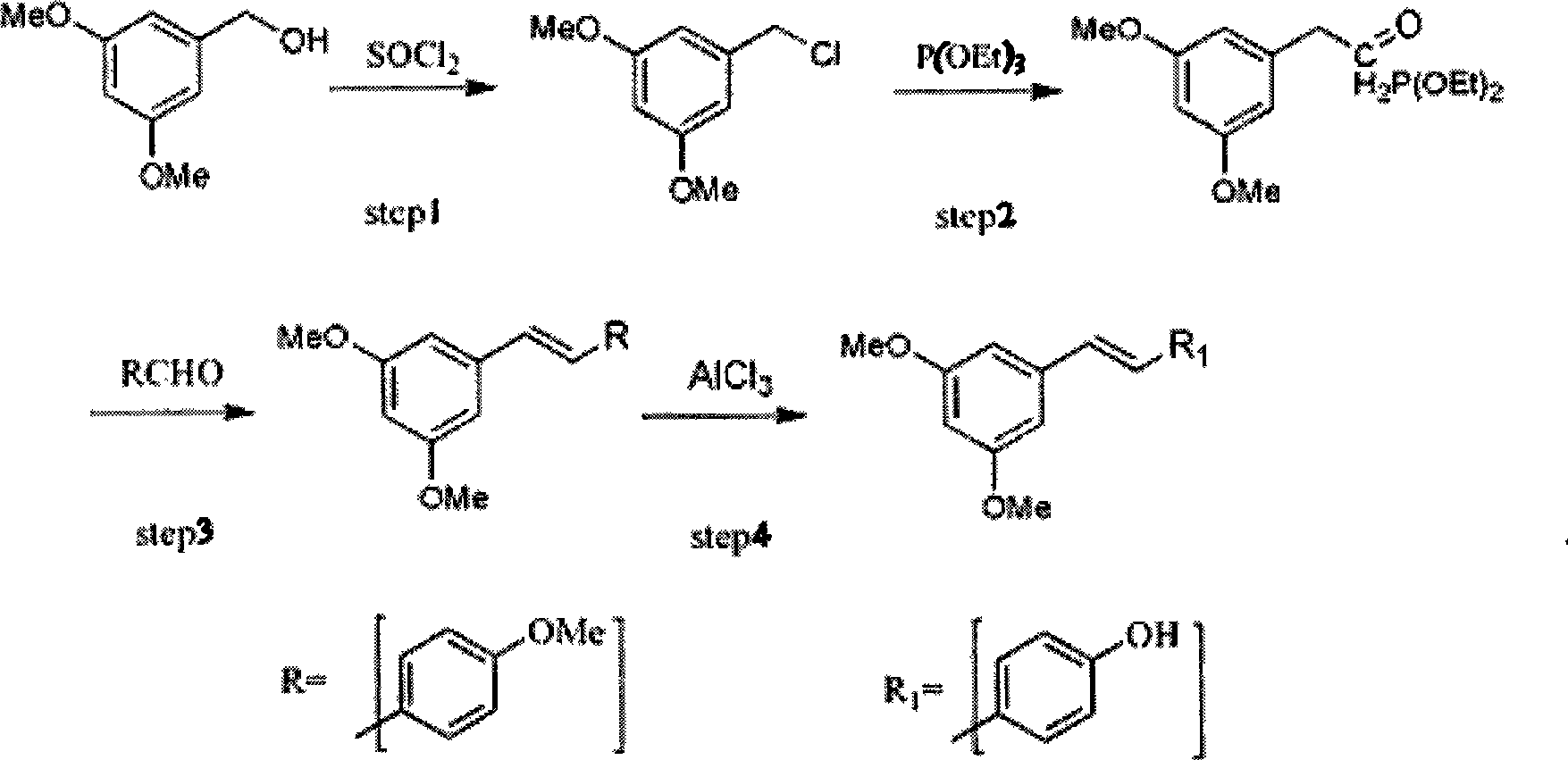
[0038] Step 2) 3) 4) is basically the same as embodiment 1, the difference is
[0039] In step 4), react at 140° C. for 6 hours; use 50 ml of ethanol: water = 1:2 mixed solvent for recrystallization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com