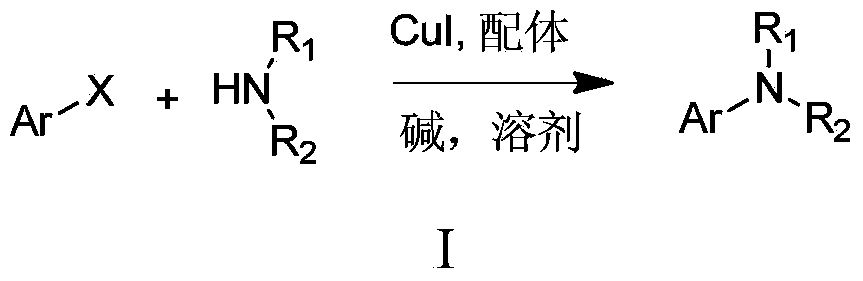
Method for N-arylation of alkane amine
A technology for alkane amine and arylation, which is applied in the field of N-arylation of alkane amine, can solve the problems of expensive ligand, difficult reaction, unfavorable industrial application and the like, and achieves good application prospect, low price and wide base The effect of the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
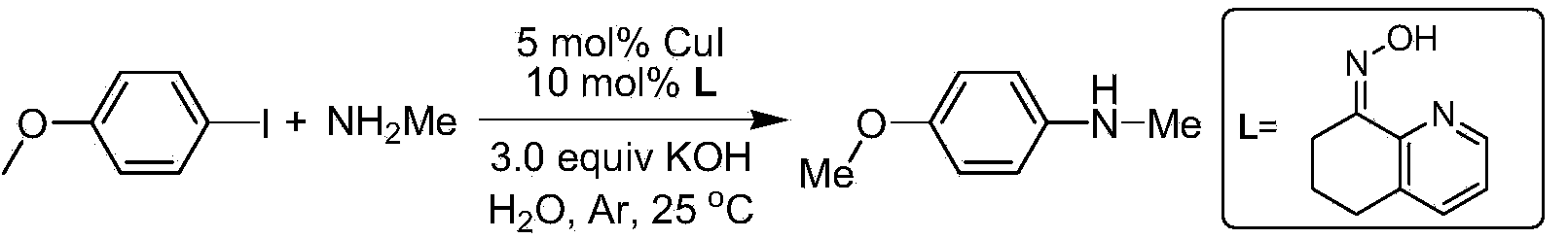
[0036] Preparation of N-methyl-4-methoxyaniline.
[0037]
[0038] In a reaction tube sealed at one end, add 234mg p-methoxyiodobenzene (MW=234,1.0mmol), then add 168mg KOH (MW=56,3mmol), 16.1mg6,7-dihydroquinoline-8 (5H )-ketoxime (i.e. ligand L) (MW=161,0.1mmol), 9.5mg CuI (MW=190,0.05mmol), 1.0ml30% methylamine aqueous solution (MW=31,9.6mmol), in argon or Under nitrogen protection, stir and react at room temperature (20-25°C) for 18 hours, extract the reaction mixture three times with 25 ml of ethyl acetate, combine and dry the extracts, distill under reduced pressure, and separate through a silica gel column (eluent petroleum ether: acetic acid Ethyl ester=8:1), obtain 133mg product N-methyl-p-methoxyaniline, productive rate 97%.
[0039] The characterization data of this product N-methyl-p-methoxyaniline is:
[0040] 1H NMR (CDCl 3 ,400MHz):δ=6.81(d,J=8.4Hz,2H),6.63(d,J=8.8Hz,2H),3.75(s,3H),2.81(s,3H);13C NMR(CDCl 3,75MHz): δ=152.4, 143.3, 114.9, 113.9, 55.9, 31.8...
Embodiment 2
[0042] Preparation of N-methyl-4-methoxyaniline.
[0043]
[0044] Using K 2 CO 3 420 mg was used as the base. According to the method described in Example 1, 4-methoxyiodobenzene (234 mg, 1.0 mmol) and 1.0 ml of 30% methylamine aqueous solution (MW=31, 9.6 mmol) were stirred at room temperature for 18 h. The crude product was purified by column chromatography (20:1 petroleum ether: ethyl acetate), yield: 47%.
[0045] The characterization data of this product N-methyl-p-methoxyaniline is:
[0046] 1H NMR (CDCl 3 ,400MHz):δ=6.81(d,J=8.4Hz,2H),6.63(d,J=8.8Hz,2H),3.75(s,3H),2.81(s,3H);13C NMR(CDCl 3 ,75MHz): δ=152.4, 143.3, 114.9, 113.9, 55.9, 31.8.
Embodiment 3
[0048] Preparation of N-methyl-4-methoxyaniline.
[0049] Using Cs 2 CO 3 975mg (3mmol) was used as the base. According to the method described in Example 1, 4-methoxyiodobenzene (234mg, 1.0mmol) and 1.0ml of 30% methylamine aqueous solution (MW=31, 9.6mmol) were stirred at room temperature for 18h. The crude product was purified by column chromatography (20:1 petroleum ether: ethyl acetate), yield: 75%.
[0050] The characterization data of this product N-methyl-p-methoxyaniline is:
[0051] 1H NMR (CDCl 3 ,400MHz):δ=6.81(d,J=8.4Hz,2H),6.63(d,J=8.8Hz,2H),3.75(s,3H),2.81(s,3H);13C NMR(CDCl 3 ,75MHz): δ=152.4, 143.3, 114.9, 113.9, 55.9, 31.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com