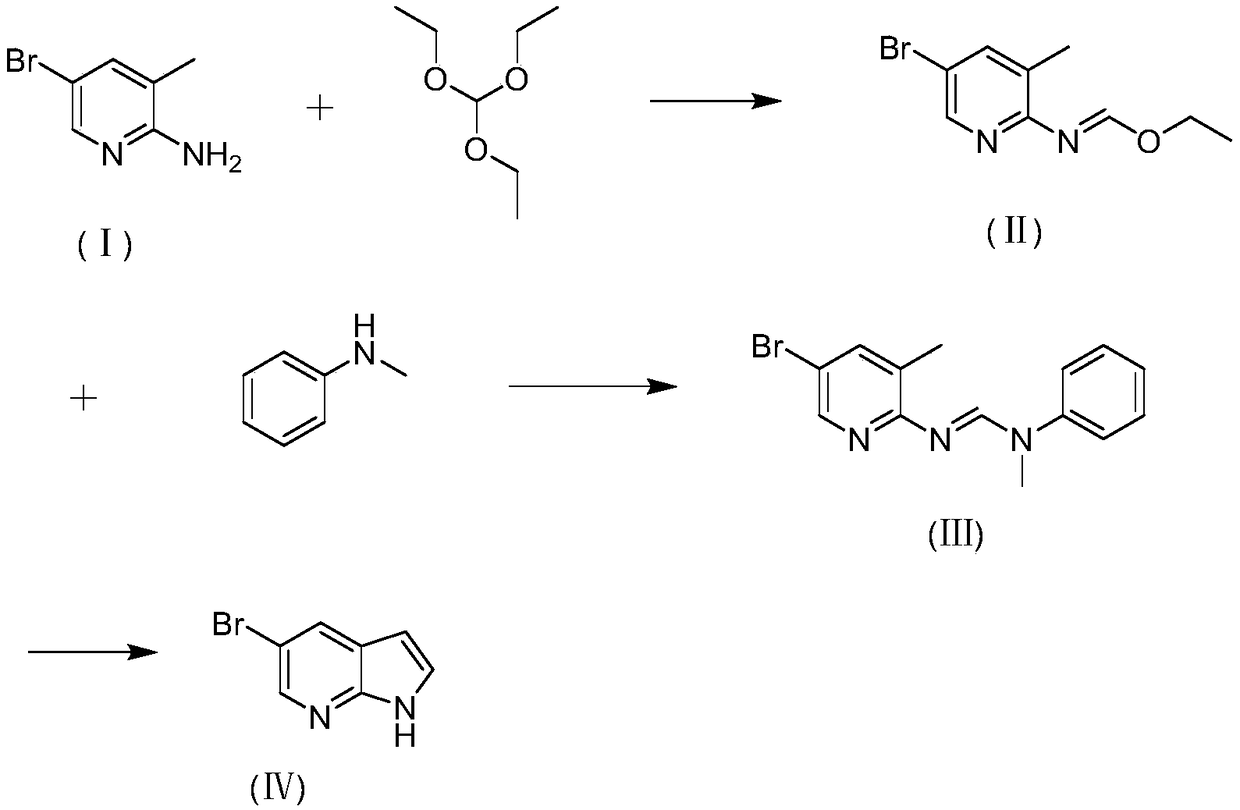
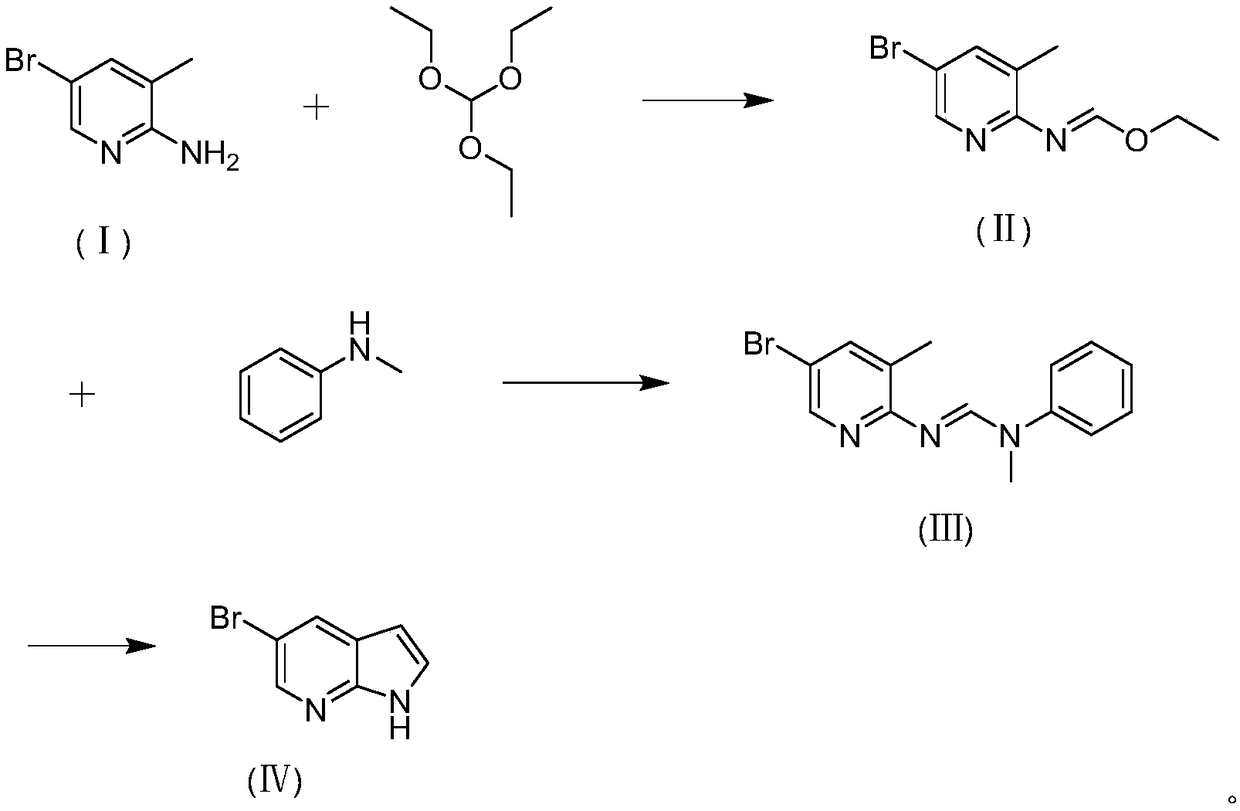
Preparation method of 5-bromine-7-azaindole
A technology of azaindole and bromopyridine, which is applied in the field of compound preparation, can solve the problems of cumbersome post-processing, heavy metal residues, and danger, and achieve the effects of shortening reaction time, easy control of conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a single-necked bottle, dissolve 1.0g of 2-amino-3-methyl-5-bromopyridine in 6.3g of triethyl orthoformate, then add 0.02g of p-toluenesulfonic acid, build a distillation device, and heat up to 85°C for reaction . The reaction was carried out for 1 hour, and TLC detected that the reaction of the raw materials was complete. Ethanol and triethyl orthoformate were distilled off under reduced pressure to obtain 1.29 g of ethyl N-(3-methyl-5-bromopyridin-2-yl)formimidate of formula (II), with a yield of 99%. Then, the obtained ethyl N-(3-methyl-5-bromopyridin-2-yl)formimidate was dissolved in 1.7 g of N-methylaniline, a distillation device was built, and the temperature was raised to 110° C. for reaction. The reaction was carried out for 2 hours, and TLC detected that the reaction of the raw materials was complete. Ethanol and triethyl orthoformate were distilled off under reduced pressure, and beating with methanol: water = 2:1 to obtain formula (Ⅲ) N-(3-methyl-5-bromo...
Embodiment 2
[0042] Under nitrogen protection, 0.19 g of sodium amide was added to 2 mL of N-methylaniline, and the temperature was raised to reflux for 30 minutes. Then 1.0g of N-(3-methyl-5-bromopyridin-2-yl)-N'-methyl-N'-phenylformamidine was dissolved in 2mL of N-methylaniline, and slowly dropped into the reaction system After 2 hours, TLC detected that the raw material had reacted completely. N-methylaniline was distilled off under reduced pressure. Water was added to quench the reaction, extracted with ethyl acetate, the organic phase was separated, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 0.39 g of 5-bromo-7azaindole of formula (IV), with a yield of 60%.
[0043] 1 H NMR (500MHz, CDCl 3) δ 10.90 (s, 1H), 8.34 (s, 1H), 8.05 (s, 1H), 7.37 (d, J=4.0Hz, 1H), 6.62 (d, J=3.0Hz, 1H).
[0044] Under nitrogen protection, 0.47 g of sodium tert-butoxide was added to 2 mL of N-methylaniline, and the temperature was raised to reflux for 30 minutes. Then 1.0g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com