Synthetic method of 4-chloro-2-(N-methyl-N-phenyl sulfamoyl) methyl benzoate
A technology of phenylaminosulfonyl and methyl benzoate, applied in the field of synthesis of 4-chloro-2-methyl benzoate, can solve the problems of high price, low product yield, long reaction steps and the like, and achieves safety Good, easy to obtain raw materials, simplified operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
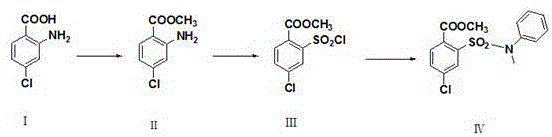
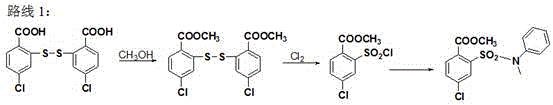
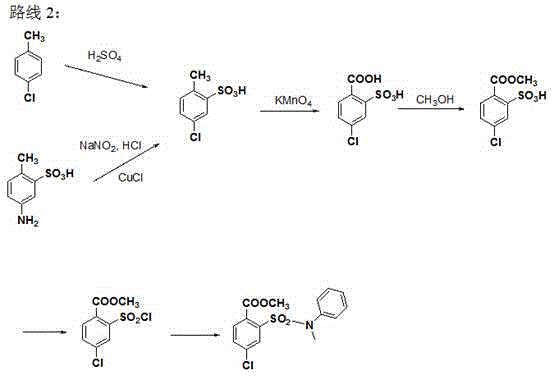
[0029] A kind of synthetic method of 4-chloro-2-(N-methyl-N-phenylsulfamoyl) benzoic acid methyl ester, comprises the steps: take 4-chloro-2-aminobenzoic acid as raw material, through esterification After the reaction, diazotization and Sandmeyer reaction were used to obtain methyl 4-chloro-2-sulfonic acid chlorobenzoate, and then condensed with N-methylaniline to obtain 4-chloro-2-(N-methyl-N- phenylsulfamoyl)benzoate methyl ester.
[0030] Its chemical reaction equation is as follows:
[0031]
Embodiment 1
[0033] In 400 grams of methanol, add 100 grams (0.58 mol) of 4-chloro-2-aminobenzoic acid, cool down in an ice-water bath, and slowly add 125 grams (1.05 mol) of thionyl chloride dropwise below 20°C, then dropwise add After completion, slowly raise the temperature to reflux, and after 12 hours of reflux reaction, concentrate under reduced pressure at 30~40°C and concentrate to dryness. Cool down to 0-5°C in an ice-water bath, without further treatment, add 300 grams of concentrated hydrochloric acid directly, add 150 grams (0.65 mol) of 30% sodium nitrite solution dropwise at 0-5°C, and continue at 0-5°C React at 5°C for 1 hour, then add 8.5 grams (0.05mol) of copper chloride, and add 120 grams of saturated sulfur dioxide in acetic acid solution dropwise at 5-10°C, and continue to react at 5-10°C for 1 hour after the dropwise addition is completed. After the reaction, add 500 g of dichloromethane and 200 g of water, stir for 15 minutes and then separate into layers, remove the...
Embodiment 2
[0035] In 400 grams of methanol, add 100 grams (0.58 mol) of 4-chloro-2-aminobenzoic acid, cool down in an ice-water bath, and slowly add 120 grams (1.00 mol) of thionyl chloride dropwise below 20°C, then dropwise add After completion, slowly raise the temperature to reflux, and after 10 hours of reflux reaction, concentrate under reduced pressure at 30~40°C and concentrate to dryness. Cool down to 0-5°C in an ice-water bath, without further treatment, add 300 grams of concentrated hydrochloric acid directly, add 150 grams (0.65 mol) of 30% sodium nitrite solution dropwise at 0-5°C, and continue at 0-5°C React at 5°C for 1 hour, then add 8.5 grams (0.05mol) of copper chloride, and add 120 grams of saturated sulfur dioxide in acetic acid solution dropwise at 5-10°C, and continue to react at 5-10°C for 1 hour after the dropwise addition is completed. After the reaction, add 500 g of dichloromethane and 200 g of water, stir for 15 minutes and then separate into layers, remove the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com