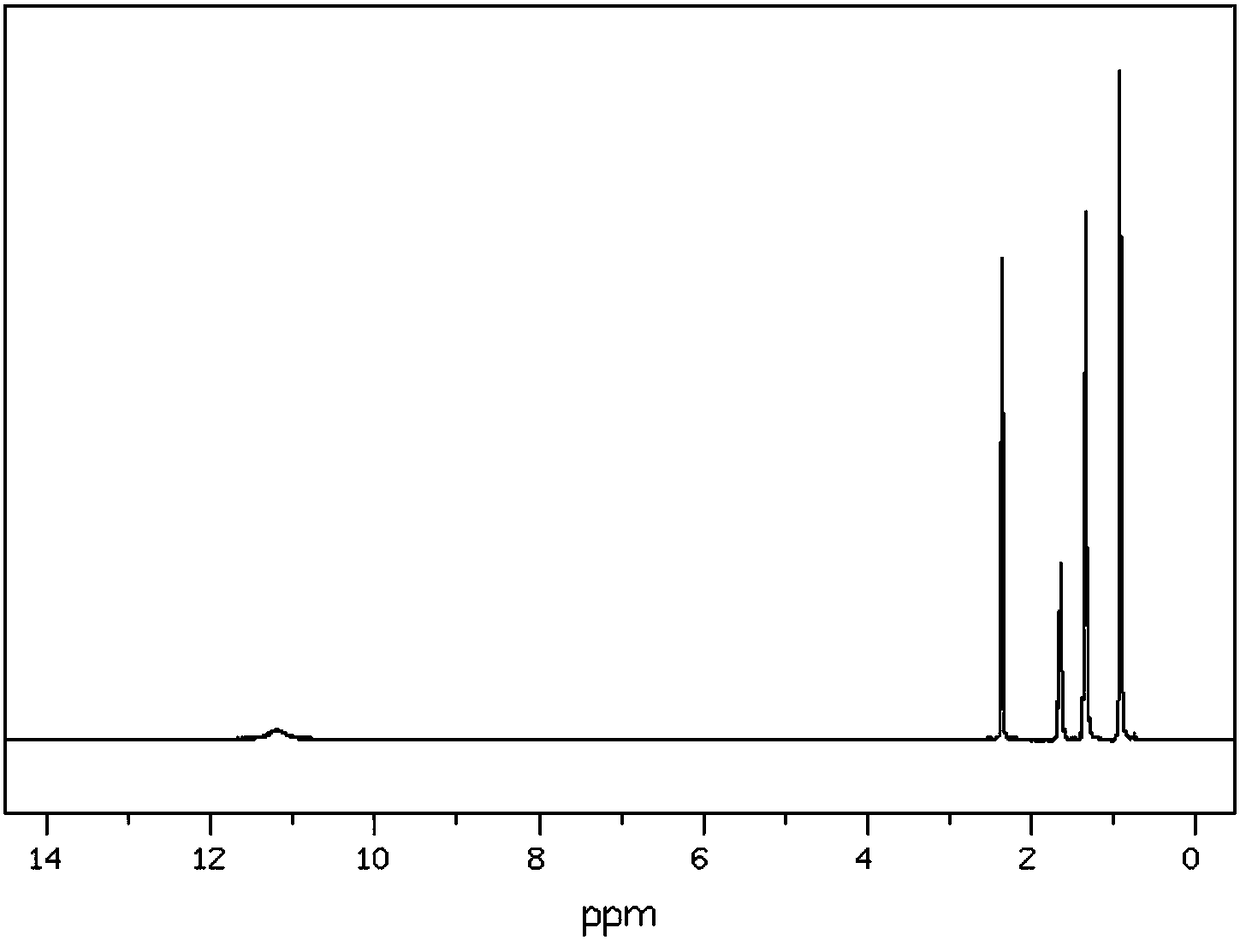
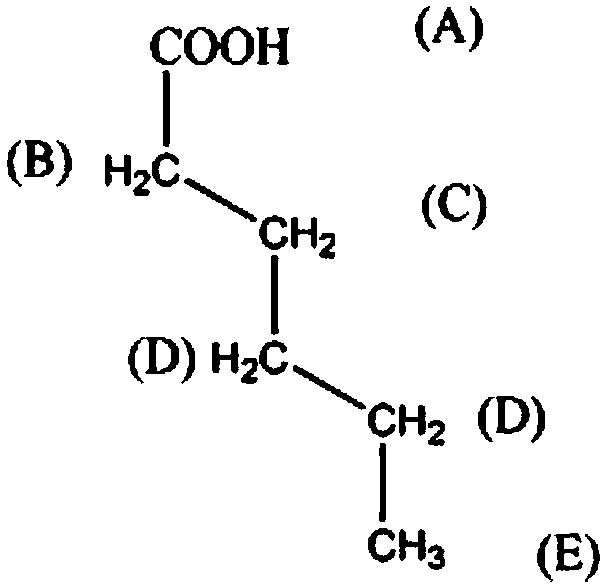
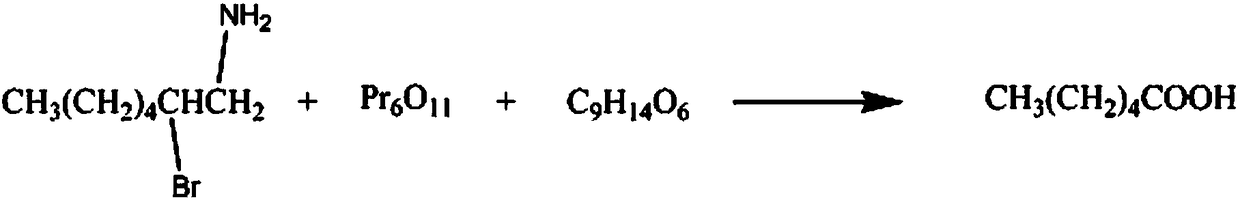Synthetic method for organic synthesis intermediate caproic acid
A technology of organic synthesis and synthesis method, which is applied in the preparation of organic intermediates and the synthesis field of organic synthesis intermediate caproic acid, can solve the problems of high requirements on equipment corrosion resistance, increased equipment maintenance costs, reduced production costs, etc. The effect of reducing equipment maintenance costs, shortening reaction time, and reducing reaction energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthetic method of organic synthesis intermediate hexanoic acid, comprises the steps:
[0019] A: Add 2mol 1-amino-2-bromo-heptane in the reaction vessel, control the stirring speed at 210rpm, add 800ml of potassium chloride solution with a mass fraction of 15% within 50min, raise the temperature to 40°C, and maintain for 30min;
[0020] B: Continue to add 2mol praseodymium oxide powder, raise the temperature to 60°C within 3h, then add 4mol mass fraction of 15% glycerol triacetate solution, continue the reaction for 60min, lower the temperature to 10°C, the solution is separated, the mass Fraction is 20% sodium nitrate solution washing 2 times, mass fraction is 40% 3-hexanone solution washing 6 times, mass fraction is 60% N-methylaniline solution washing 4 times, mass fraction is 80% Recrystallized in pentaerythritol solution and dehydrated with anhydrous calcium sulfate dehydrating agent to obtain 206.48 g of hexanoic acid as a finished product with a yield of 89...
Embodiment 2
[0022] The synthetic method of organic synthesis intermediate hexanoic acid, comprises the steps:
[0023] A: Add 2mol 1-amino-2-bromo-heptane in the reaction vessel, control the stirring speed at 230rpm, add 800ml of potassium chloride solution with a mass fraction of 18% within 60min, raise the temperature to 43°C, and maintain for 40min;
[0024] B: Continue to add 2.5mol praseodymium oxide powder, raise the temperature to 63.5°C within 3.5h, then add 4.5mol mass fraction of 18% glycerin triacetate solution, continue the reaction for 75min, lower the temperature to 12.5°C, and divide the solution layer, mass fraction is 23.5% sodium nitrate solution washes 3 times, mass fraction is 43% 3-hexanone solution washes 7 times, mass fraction is 62.5% N-methylaniline solution washes 5 times, in mass fraction Recrystallize in 83.5% pentaerythritol solution and dehydrate with anhydrous potassium carbonate dehydrating agent to obtain 213.44 g of hexanoic acid as a finished product wit...
Embodiment 3
[0026] The synthetic method of organic synthesis intermediate hexanoic acid, comprises the steps:
[0027] A: Add 2mol 1-amino-2-bromo-heptane in the reaction vessel, control the stirring speed at 250rpm, add 800ml of potassium chloride solution with a mass fraction of 21% within 70min, raise the temperature to 46°C, and maintain it for 50min;
[0028] B: Continue to add 3mol praseodymium oxide powder, raise the temperature to 67°C within 4h, then add 5mol mass fraction of 21% triacetin solution, continue the reaction for 90min, lower the temperature to 15°C, the solution is separated, and the mass Fraction is 27% sodium nitrate solution washes 4 times, mass fraction is 46% 3-hexanone solution washes 8 times, mass fraction is 65% N-methylaniline solution washes 6 times, in mass fraction is 87% Recrystallized in pentaerythritol solution and dehydrated with anhydrous calcium sulfate dehydrating agent to obtain 222.72 g of hexanoic acid as a finished product with a yield of 96%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com