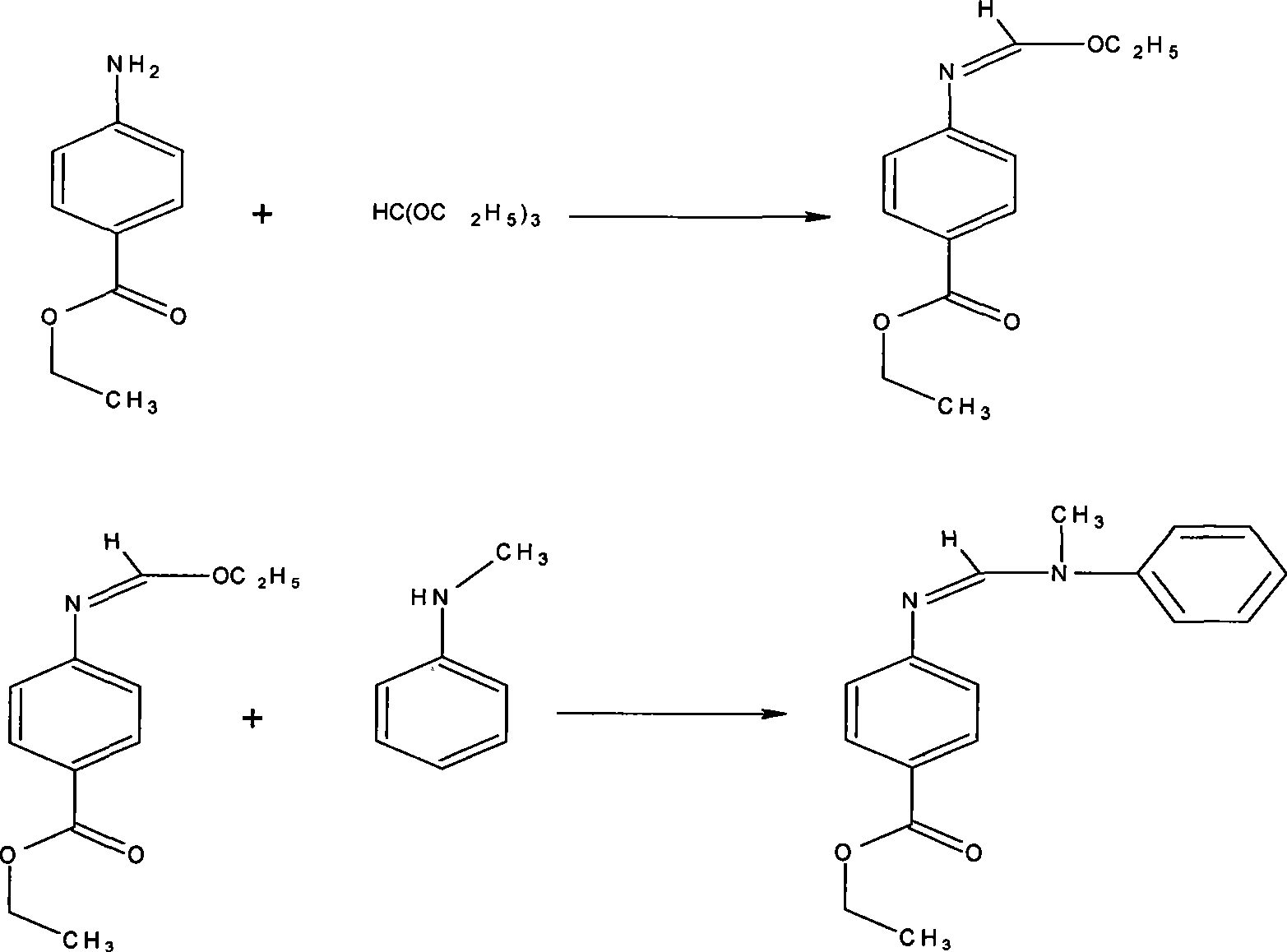
Preparation of N-(4-ethoxy carbonyl phenyl)-N'-methyl-N'-phenyl formamidine
A technology of ethoxycarbonylphenyl and phenylformamidine is applied in the field of preparation of N--N'-methyl-N'-phenylformamidine, which can solve the problems of cumbersome separation and purification, color and purity influence, etc. , to achieve the effect of good product color, easy large-scale industrial production, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Put 10Kg of ethyl p-aminobenzoate and 10Kg of triethyl orthoformate into a 50L reaction kettle, turn on the heating, raise the temperature to 100°C, ethanol is generated, after the reaction is completed, turn on the vacuum pump, distill under reduced pressure, and evaporate the remaining raw materials. Triethyl formate, and then collect the intermediate fraction to obtain 12Kg of yellow-green oily liquid, then all of it is pumped into a 50L reactor, and then 10Kg of N-methylaniline is added, and the temperature is slowly raised to 100-150°C (slightly reduced pressure, with ethanol is taken out), after the reaction finishes, underpressure distillation collects the remaining N-methylaniline cuts, and then collects the product cuts to obtain 12.4Kg slightly yellow N-(4-ethoxycarbonylphenyl)-N' -Methyl-N'-phenylformamidine product, content 99.2%.
Embodiment 2
[0018] Put 10Kg of ethyl p-aminobenzoate and 30Kg of triethyl orthoformate into a 50L reactor, turn on the heating, raise the temperature to 150°C, and ethanol will be generated. Triethyl formate, and then collect the intermediate fraction to obtain 13Kg of yellow-green oily liquid, then all of it is pumped into a 50L reaction kettle, and then 25Kg of N-methylaniline is added, and the temperature is slowly raised to 100-150°C (slightly reduced pressure, with ethanol is taken out), after the reaction finishes, vacuum distillation collects the remaining N-methylaniline cuts, and then collects the product cuts to obtain 15Kg slightly yellow N-(4-ethoxycarbonylphenyl)-N'- Methyl-N'-phenylformamidine product, content 99.2%.
Embodiment 3
[0020] Put 10Kg of ethyl p-aminobenzoate and 20Kg of trimethyl orthoformate into a 50L reactor, turn on the heating, raise the temperature to 100°C, methanol will be generated, after the reaction, turn on the vacuum pump, and distill under reduced pressure to remove the remaining orthoformic acid Trimethyl ester, then collect the intermediate fraction to obtain 11.5Kg yellow-green oily liquid, then all of it is pumped into a 50L reaction kettle, and then 50KgN-methylaniline is dropped into, and the temperature is slowly raised to 100~150°C (slightly decompressed, with Methanol is taken out), after the reaction finishes, underpressure distillation collects the remaining N-methylaniline cuts, and then collects the product cuts to obtain 14.6Kg slightly yellow N-(4-ethoxycarbonylphenyl)-N' -Methyl-N'-phenylformamidine product, content 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com