Preparation method of compound 19-desmethyl-4-androstene-3,17 diketone
The technology of a compound and androstene, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as limiting the scope of use of hypochlorite oxidants, difficulty in storage and transportation of hypochlorite solution, and the process formula is easily affected by weather conditions. Storage and transportation, good stability, and the effect of improving product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
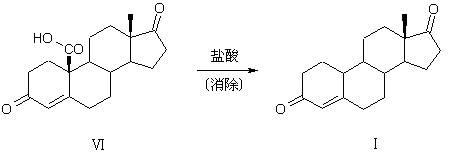
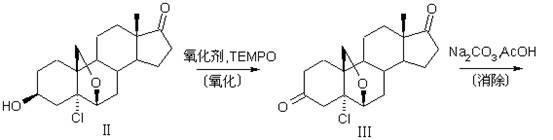
[0040] The preparation method of compound 19-desmethyl-4-androstene-3,17-dione (I) of the present invention uses compound 5α-chloro-3β-hydroxyl-6β, 19β-epoxy-androstene-17- Ketone (Ⅱ) is raw material, comprises the following steps:
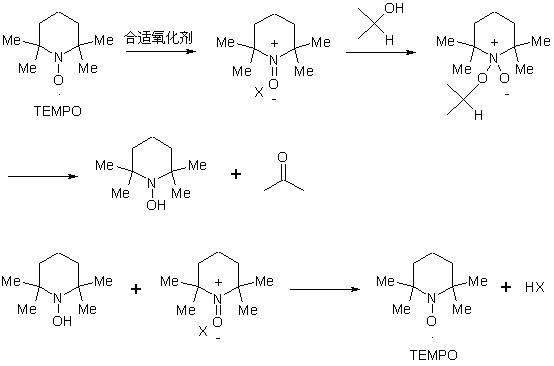
[0041] The first step, the oxidation reaction:
[0042]Compound 5α-chloro-3β-hydroxyl-6β, 19β-epoxy-androst-17-one (Ⅱ) is added to haloalkane, alkaline buffer solution is added, and catalytic amount of 2,2,6,6-tetramethyl Piperidine-N-oxide (TEMPO), adjust the temperature to 5-15°C, add N-halogenated amide oxidant, stir and react at 5-15°C for 4-6 hours; after the reaction, add a reducing agent to quench the oxidation , separate layers, wash the organic layer until clean, separate the layers, concentrate the organic layer under reduced pressure, add acetone to take out the halogenated alkanes, add water, concentrate until there is no acetone smell, cool to 5-10 ° C, filter, wash until neutral, and obtain compound 5α -Chloro-6β,19β-epoxy-androst-...
Embodiment 1
[0062] The preparation process of compound 19-hydroxyl-4-androstene-3,17-dione (Ⅴ):
[0063] (1) Oxidation reaction: In the reaction bottle, put the compound 5α-chloro-3β-hydroxy-6β, 19β-epoxy-androst-17-one (II) 100g, dichloromethane 1000ml, stir to dissolve, add sodium carbonate - Sodium bicarbonate solution 1000ml, add 2,2,6,6-tetramethylpiperidine-N-oxide (TEMPO) 0.93g, adjust the temperature to 5~10℃, add 50g dichlorodimethylhydantoin, Stir and react at 5 to 10°C for 6 hours; after the reaction is complete, add sodium metabisulfite to quench the oxidation, separate layers, wash the organic layer until clean, separate layers, concentrate the organic layer under reduced pressure, add acetone to take out dichloromethane, add water, and concentrate to No acetone smell, cool to 5-10°C, filter, wash until neutral to obtain compound 5α-chloro-6β, 19β-epoxy-androst-3,17-dione (Ⅲ) wet product;
[0064] (2) Elimination, reduction, and ring-opening reactions: In the reaction bottle...
Embodiment 2
[0066] The preparation process of compound 19-hydroxyl-4-androstene-3,17-dione (Ⅴ):
[0067] (1) Oxidation reaction: In the reaction bottle, put the compound 5α-chloro-3β-hydroxy-6β, 19β-epoxy-androst-17-one (II) 100g, chloroform 1000ml, stir to dissolve, add potassium carbonate - Potassium bicarbonate solution 1000ml, add 2,2,6,6-tetramethylpiperidine-N-oxide (TEMPO) 1.0g, adjust the temperature to 10~15℃, add dibromodimethylhydantoin 100g, Stir and react at 10-15°C for 4 hours; after the reaction, add sodium bisulfite to quench the oxidative property, separate layers, wash the organic layer until clean, separate layers, concentrate the organic layer under reduced pressure, add acetone to take out chloroform, add water, Concentrate until there is no acetone smell, cool to 5-10°C, filter, and wash until neutral to obtain the wet product of compound 5α-chloro-6β, 19β-epoxy-androst-3,17-dione (Ⅲ);
[0068] (2) Elimination, reduction, and ring-opening reactions: In the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com