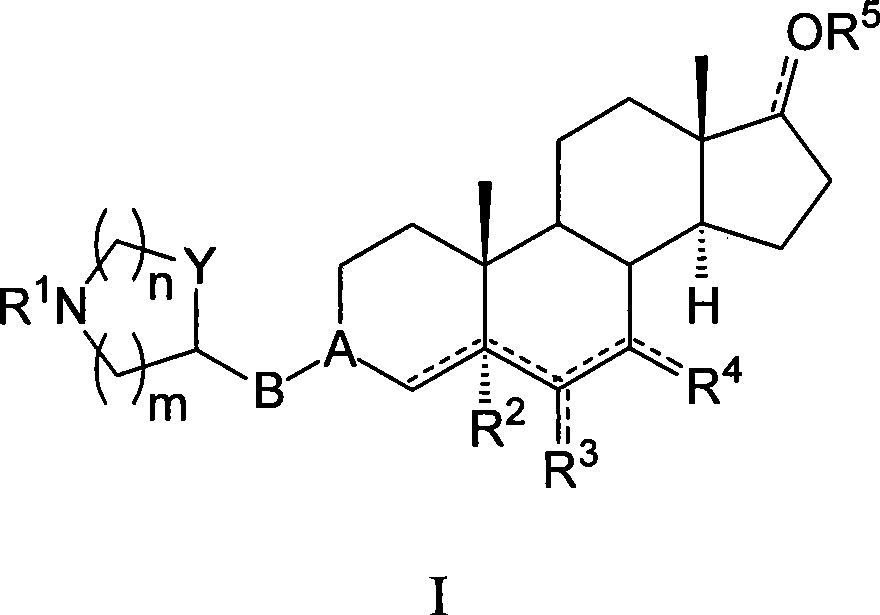
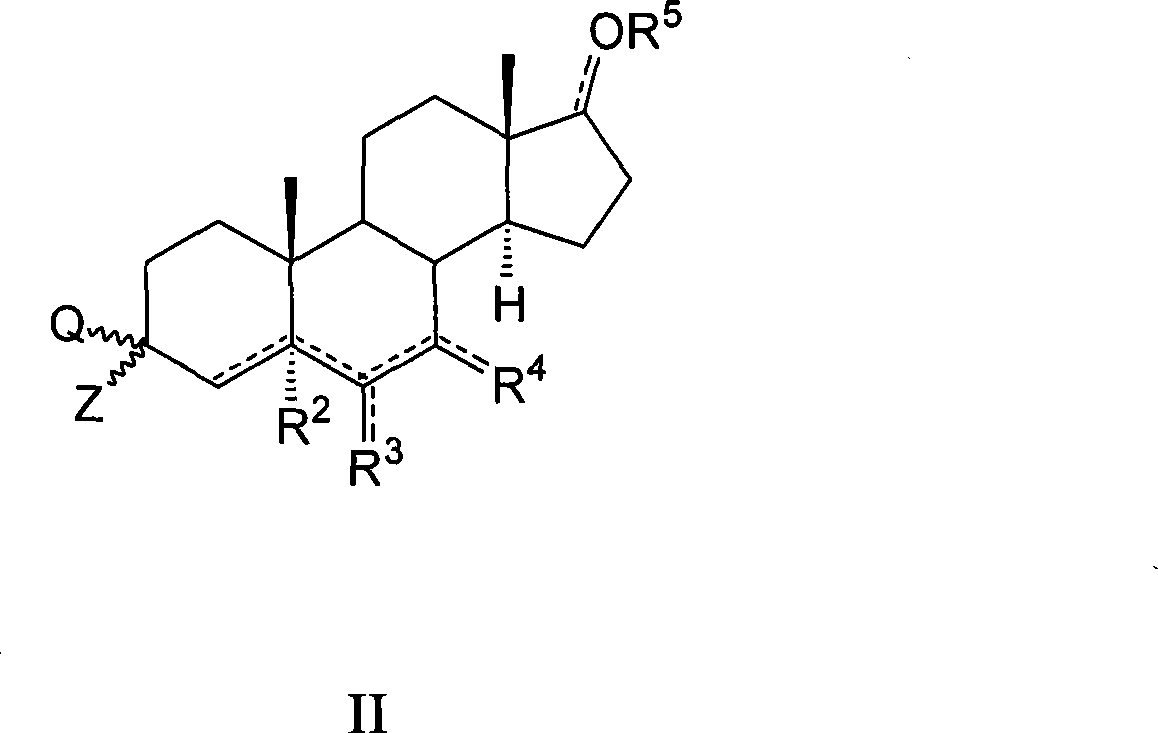
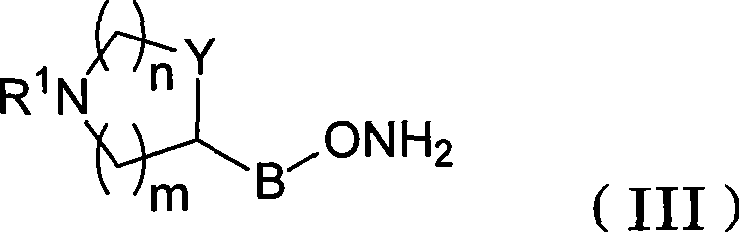Azaheterocyclyl derivatives of androstanes and androstenes as medicaments for cardiovascular disorders
A compound, branched chain alkyl technology, applied in the field of new 3-position nitrogen heterocyclic derivatives, can solve side effects and other problems, and achieve high efficacy, long duration of action, and good therapeutic ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0299] (E) 3-(4-piperidinyl)oxyiminoandrostane-6,17-dione hydrochloride (I-aa)
[0300] A solution of androstane-3,6,17-trione (160 mg) in THF (3.2 mL) was added to 4-piperidinyloxyamine dihydrochloride (III-a, Preparation 1, 100 mg) and Na 2 HPO 4 12H 2 A solution of O (380 mg) in water (1.6 mL). After 2 hours at room temperature, NaCl (150 mg) was added and stirred for 15 minutes. The mixture was extracted with THF (2 x 2 mL), and the combined organic phases were washed with brine (3 x 3 mL), washed with Na 2 SO 4 Dry and evaporate to dryness. The residue was analyzed by flash column chromatography (SiO 2 , CH 2 Cl 2 :MeOH:NH3 9:1:0.1) purification. 5M HCl / EtOAc was added to the concentrated fraction. with Et 2 After O dilution, the solid was collected by filtration to afford the title compound I-aa (140 mg, 60%). 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 8.68 (2H, bb), 4.17 (1H, m), 3.15-2.90 (5H, m), 2.60-1.10 (23H, m), 0.79 (3H, s), 0.78 (3H, s).
example 2
[0302] (E, Z) 3-(3-azetidine)oxyiminoandrostane-6,17-dione fumarate (I-ab)
[0303] Following the procedure described in Example 1, starting from androstane-3,6,17-trione (950 mg) and 3-azetidinoxamine dihydrochloride (III-b, Preparation 2,500 mg), The title compound I-ab was obtained as a white solid (1.21 g, 80%). 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 6.50 (2H, s), 4.87 (1H, m), 4.10-2.90 (5H, m), 2.50-1.20 (19H, m), 0.79 (6H, s).
example 3
[0305] (E) 3-[3-(RS)-pyrrolidinyl]oxyiminoandrostane-6,17-dione hydrochloride (I-ac)
[0306] 3-(RS)-Pyrrolidinyloxyamine dihydrochloride (III-c, Preparation 3, 227 mg) was mixed with androstane-3,6,17-trione (495 mg) in THF:water (2 / 1 , 27 mL) solution was stirred for 30 minutes. NaCl was added and stirred until the two phases separated. After extracting the aqueous layer with THF, the combined organic phases were washed with brine, dried and evaporated. Flash column chromatography (SiO 2 , CH 2 Cl 2 :MeOH:NH 3 9:1:0.1) method for purification. To this concentrated fraction was added 5M HCl in EtOAc. After dilution with Et2O, the solid was collected by filtration to afford the title compound I-ac (464 mg, 60%). 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 9.59 (1H, bb), 9.41 (1H, bb), 4.74 (1H, m), 3.80-2.90 (5H, m), 2.60-1.20 (21H, m), 0.78 (6H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com