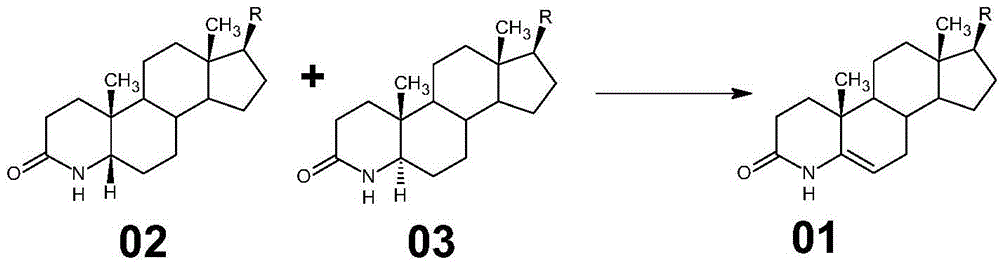
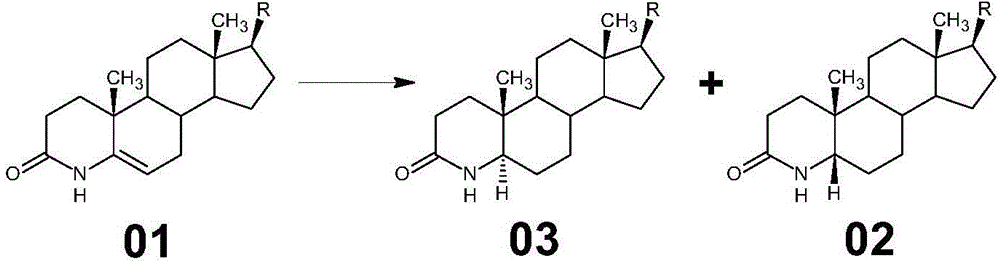
Method for preparing 3-carbonyl-4-aza-5-androstene-17 beta carboxylic acid derivative from mother solution reclaimed materials of hydrogenation reaction
A technology for recovering carboxylic acid derivatives and mother liquor, applied in steroids, organic chemistry, etc., can solve problems such as waste of the environment, pollution, etc., and achieve the effect of reducing pollution and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Dehydrogenation reaction to prepare methyl 3-carbonyl-4-aza-5-androstene-17βcarboxylate 01
[0057] The preparation method comprises the following steps:
[0058] (1) Hydrogenation of 3-carbonyl-4-aza-5-androstene-17β carboxylic acid derivative 01 to prepare the mother liquor of 3-carbonyl-4-aza-5α-androstene-17β carboxylic acid derivative 03 The reclaimed material is used as a raw material, and the raw material is dissolved in glacial acetic acid, a palladium carbon catalyst is added, oxygen is passed through, pressurized, and the temperature is raised to carry out a dehydrogenation reaction;
[0059] The specific operation method is: at room temperature, in a 2000ml stainless steel hydrogenation reactor equipped with a thermometer and a stirrer, add glacial acetic acid 1200ml, stir, add the hydrogenation mother liquor reclaimed material (3-carbonyl- 4-aza-5α-androsta-17β-carboxylate methyl ester (03) and 3-carbonyl-4-aza-5β-androsta-17β-carboxylate methyl e...
Embodiment 2
[0068] Identical with embodiment 1, difference only is: in step (1), described palladium carbon catalyst is the palladium carbon that mass content is 1%; Described hydrogenation mother liquor reclaimed material, glacial acetic acid and palladium carbon catalyst are 1 by mass ratio: 8:0.1; the oxygen pressure is 0.2MPa; the reaction temperature is 120°C; the reaction time of the dehydrogenation reaction is 18 hours. In step (3), methanol is added to the system, and the mass ratio of the hydrogenation mother liquor reclaimed material to methanol is 1:1.
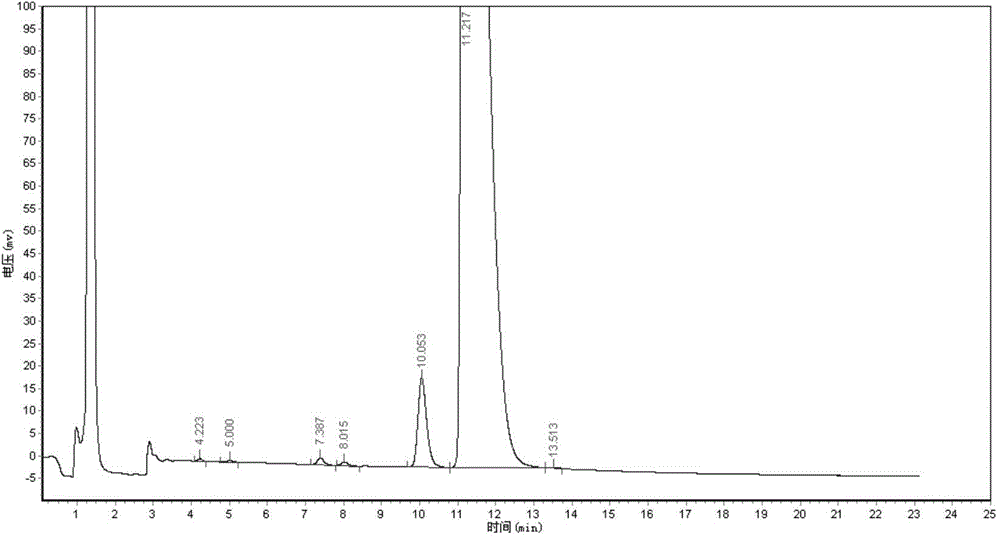
[0069] HPLC detection: the purity of 01 is 95.2623%; the content of 03 is 0.2457%.
Embodiment 3
[0071] Identical with embodiment 1, difference only is: in step (1), described palladium carbon catalyst is the palladium carbon that mass content is 25%; Described hydrogenation mother liquor reclaimed material, glacial acetic acid and palladium carbon catalyst are 1 by mass ratio: 20:0.3; the oxygen pressure is 0.8 MPa; the reaction temperature is 160° C.; the reaction time of the dehydrogenation reaction is 30 hours. In step (3), methanol is added to the system, and the mass ratio of the hydrogenation mother liquor reclaimed material to methanol is 1:3.
[0072] HPLC detection: the purity of 01 is 96.1524%; the content of 03 is 0.2916%.
[0073] Comparative experiment Preparation of methyl 3-carbonyl-4-aza-5-androstene-17βcarboxylate (01)
[0074] The specific operation method is:
[0075] At room temperature, in a 1000ml glass reaction flask equipped with a thermometer and a stirrer, add 600ml of the obtained filtrate obtained from the filtrate to be concentrated after f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com