Synthesis method of 17alpha-hydroxyl progesterone
The technology of a hydroxyprogesterone and a synthetic method, which is applied in the field of synthesis of steroid hormone drugs, can solve problems such as limited application, and achieve the effects of low raw material cost, mild reaction conditions, and easy industrial implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
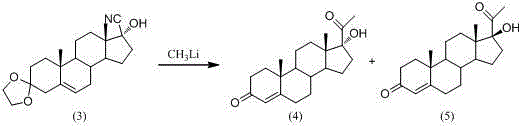
[0028] The synthetic method of 17α-hydroxyprogesterone of the present invention, with easy-to-obtain, cheap 4-androstene-dione as starting material, through liquid reagent acetone cyanohydrin cyanation, with triethyl orthoformate and ethanol protection 3 The carbonyl group is protected by vinyl butyl ether at the 17th hydroxyl group. After the Grignard reaction, it is hydrolyzed to generate important raw materials and key intermediates for the synthesis of steroid hormone drugs such as medroxyprogesterone, prednisolone, and dexamethasone. The 17α-hydroxyprogesterone; Specifically comprise the following steps:
[0029] The first step, cyanation: add acetone cyanohydrin to the weak alkaline mixed solution of 4-androstene-dione and methanol (such as sodium carbonate, potassium carbonate, sodium hydroxide), and use acetone cyanohydrin to 4-androstene The carbonyl of ene-dione (I) is cyanated to generate compound 17α-hydroxy-17β-cyanoandrost-4-en-3-one (II);
[0030]
[0031] I...
Embodiment 1
[0043] Synthesis of compound (II)
[0044] Add 30 grams of 4-androstene-dione, 100 milliliters of methanol, 35 milliliters of acetone cyanohydrin, and 20 milliliters of 2.2% sodium carbonate solution into 250 milliliters of three-necked reaction flask equipped with mechanical stirring and a thermometer, and react at 50°C for 28 hours. The reaction was monitored by thin-layer analysis; after the reaction, it was added to 300 ml of ice water, stirred for 0.5 hours, filtered with suction, washed with water until neutral, and dried at 65°C to obtain 30.9 g of the product, with a yield of 103%.
Embodiment 2
[0046] Synthesis of compound (II)
[0047] Add 30 grams of 4-androstene-dione, 100 milliliters of methanol, 35 milliliters of acetone cyanohydrin, and 20 milliliters of 2.2% potassium carbonate solution into 250 milliliters of three-necked reaction flask equipped with mechanical stirring and a thermometer, and react for 28 hours at 50°C. The reaction was monitored by thin-layer analysis; after the reaction, it was added to 300 ml of ice water, stirred for 0.5 hours, filtered with suction, washed with water until neutral, and dried at 65°C to obtain 31.2 g of the product, with a yield of 104%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com