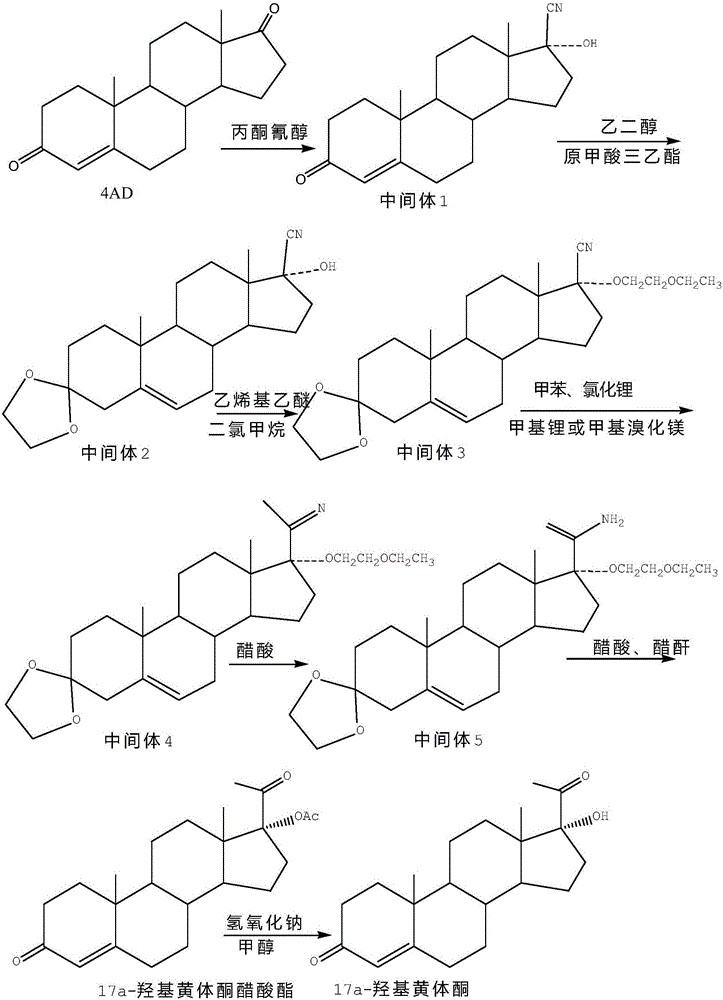
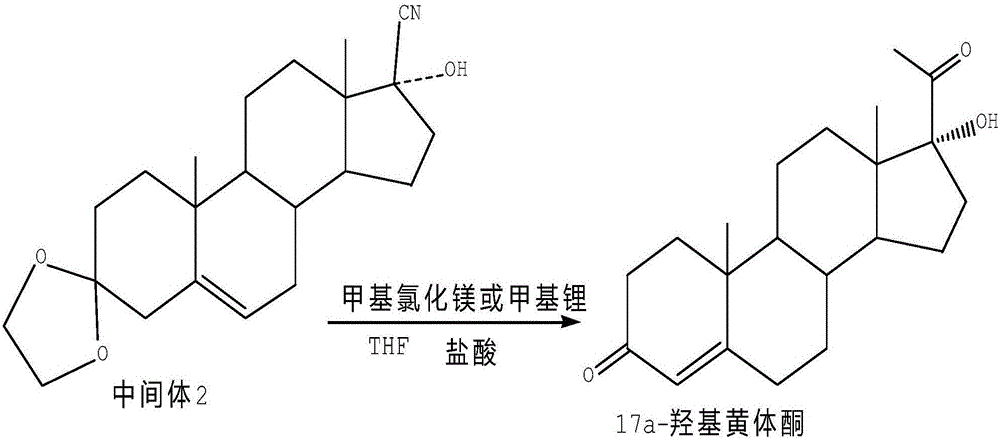
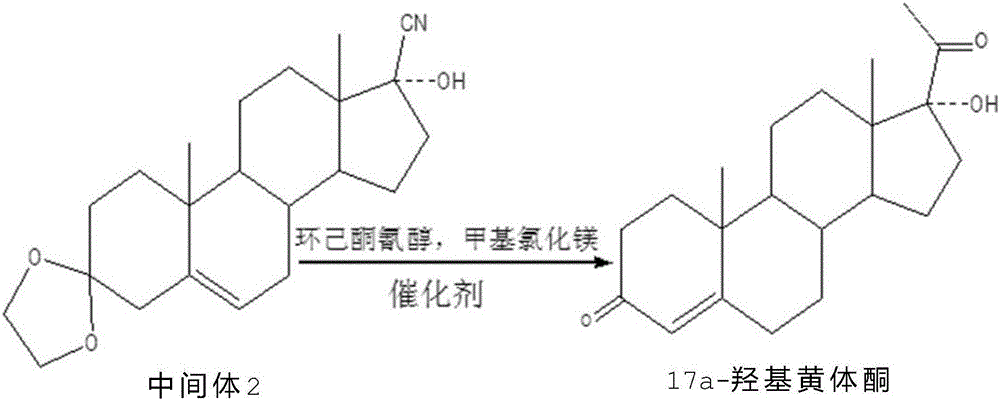
Method for synthesizing 17alpha-hydroxyprogesterone
A technology of hydroxyprogesterone and its synthetic method, which is applied in the field of preparation of steroid hormone drug intermediates, can solve the problems such as difficult inhibition of methyltestosterone, and achieve the effects of facilitating industrial production, improving product quality, and making the preparation process economical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 20g of intermediate II in 80ml of toluene, add 1g of cyclohexanone cyanohydrin and 0.2g of anhydrous lithium chloride, stir and cool down to -20~-15°C, control the temperature at -15~-10°C at 3 Add 140ml of 2M methylmagnesium chloride toluene solution dropwise within ~4 hours, and keep it warm for 3~5 hours after dropping, until TLC shows that the raw materials are completely reacted; after the reaction, control the temperature below 5°C and slowly add 60ml of 20% ammonium chloride solution, Destroy the excess Grignard reagent, then concentrate toluene under reduced pressure, cool down to 0-10°C, filter, wash with water until neutral, add the obtained intermediate product directly to 160ml of methanol, then add 6ml of concentrated hydrochloric acid, and react at 15-35°C After 2 to 3 hours, after TLC confirms that the reaction is complete, slowly add 0.6g of weak base to neutralize until the pH value is 6.0 to 6.5, and evaporate 90% of the solvent; add 60g of tap...
Embodiment 2
[0027] Dissolve 20g of intermediate II in 80ml of toluene, add 1g of cyclohexanone cyanohydrin and 0.2g of chlorinated ketone, stir and cool down to -20~-15°C, and control the temperature at -15~-10°C at 3~ Add 140ml of 2M methylmagnesium chloride toluene solution dropwise within 4 hours, and keep it warm for 3 to 5 hours after dropping, until TLC shows that the raw materials are completely reacted; Excess Grignard reagent, then concentrated toluene under reduced pressure, cooled to 0-10°C, filtered, washed with water until neutral, the obtained intermediate product was directly added to 160ml of methanol, then 6ml of concentrated hydrochloric acid was added, and the reaction was performed at 15-35°C for 2 ~ 3 hours, after TLC confirms that the reaction is complete, slowly add 0.6g of weak base, neutralize until the pH value is 6.0 ~ 6.5, evaporate 90% of the solvent; add 60g of tap water, stir and cool to 5 ~ 10 ° C, and precipitate crystallization for 1 to 2 hours, filtered,...
Embodiment 3
[0030] Dissolve 20g of intermediate II in 80ml of toluene, add 1g of cyclohexanone cyanohydrin and 0.2g of anhydrous magnesium chloride, stir and cool down to -20~-15°C, control the temperature at -15~-10°C at 3~4 Add 140ml of 2M methylmagnesium chloride tetrahydrofuran solution dropwise within 1 hour, and keep it warm for 3 to 5 hours after dropping, until TLC shows that the raw materials are completely reacted; after the reaction, control the temperature below 5°C and slowly add 60ml of 20% ammonium chloride solution to destroy excess Grignard reagent, then concentrated under reduced pressure to get tetrahydrofuran, cooled to 0-10°C, filtered, washed with water until neutral, the obtained intermediate product was directly added to 160ml of methanol, then added 6ml of concentrated hydrochloric acid, and reacted at 15-35°C for 2- After 3 hours, TLC confirms that the reaction is complete, slowly add 0.6g of weak base, neutralize until the pH value is 6.0-6.5, evaporate 90% of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com