Preparation of alpha-tetralone by two-step catalysis method
A technology of tetralone and tetralone, which is applied in the field of preparation of α-tetralone by a two-step catalytic method, can solve problems such as difficult separation, low selectivity of α-tetralone, and toxic dosage of catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
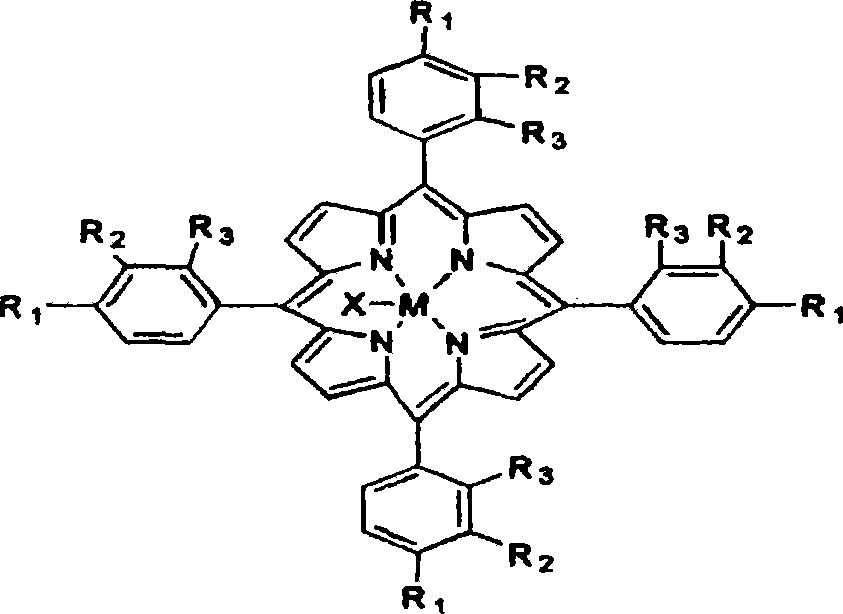
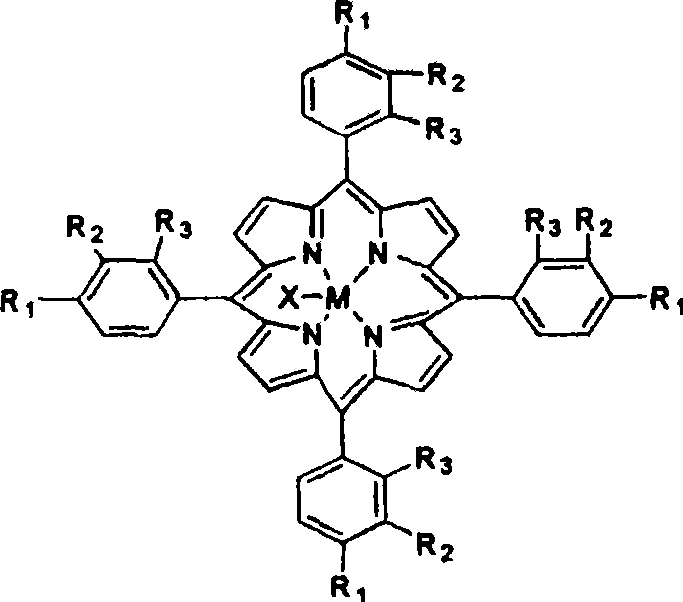
[0013] The metalloporphyrin with general formula (I) structure is dissolved in the 50ml tetralin, and the concentration of metalloporphyrin is 14.75ppm, R 1 = Cl, R 2 =R 3 =H, M=Fe, X=Cl. Air was introduced at a gas velocity of 300ml / min. React at 90 DEG C for 8 hours, the conversion rate of tetralin is 15.32%, the selectivity of α-tetralin hydroperoxide is 89.88%, the total selection of α-tetralin hydroperoxide and α-tetralone The sex is 95.37%. Take 24.40g of oxidizing solution, drop into 30ml FeSO 4 ·7H 2 Aqueous solution of O (dissolved with 0.86g of FeSO 4 ·7H 2 O), ice-water bath controls the temperature in the reaction process at about 0°C, and adds dropwise under stirring, and after 30 minutes, the dropwise addition is completed, and continues to stir and stir for 60 minutes. Hydrogen peroxide is almost completely decomposed into α-tetralone, and the total selectivity of α-tetralone is 95.31%.
Embodiment 2
[0015] The metalloporphyrin with general formula (I) structure is dissolved in the 50ml tetralin, and the concentration of metalloporphyrin is 14.75ppm, R 1 = Cl, R 2 =R 3 =H, M=Co, X=Cl. Feed into air with 300ml / min gas velocity, react at 90 ℃ for 8 hours, the transformation rate of tetralin is 34.22%, and α-tetrahydronaphthalene hydroperoxide selectivity is 83.36%, α-tetralin hydroperoxide and α-tetralone with an overall selectivity of 95.40%. Take 28.94g of oxidizing solution, drop into 30ml of CuCl aqueous solution (dissolved with 0.36g of CuCl), control the temperature in the reaction process at about 0°C in an ice-water bath, add drop by drop under stirring, after 30min dropwise addition, continue to stir for 60min, After vacuum filtration and separation treatment, the α-tetralin hydroperoxide in the oxidation solution is almost completely decomposed into α-tetralone, and the total selectivity of α-tetralone is 94.66%.
Embodiment 3
[0017] The metalloporphyrin with general formula (I) structure is dissolved in the 50ml tetralin, and the concentration of metalloporphyrin is 29.50ppm, R 1 = Cl, R 2 =R 3 =H, M=Co, X=Cl. Feed into air with 300ml / min gas velocity, react 4 hours at 90 ℃, the transformation rate of tetralin is 25.02%, and the α-tetralin hydroperoxide selectivity is 83.09%, α-tetralin hydroperoxide and α-tetralone with an overall selectivity of 94.27%. Take 25.00g of oxidizing solution, drop into 30ml of FeSO 4 ·7H 2 O aqueous solution (dissolved with 0.9g of FeSO 4 ·7H 2 0), the ice-water bath controls the temperature in the reaction process at about 0°C, and it is added dropwise under stirring, and the dropwise addition is completed in 30 minutes, and the stirring is continued for 60 minutes. Hydrogen oxide is almost completely decomposed into α-tetralone, and the total selectivity of α-tetralone is 94.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com