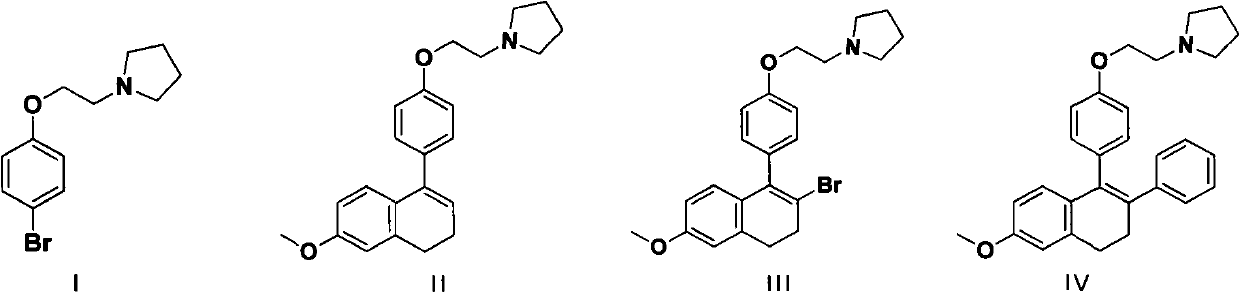
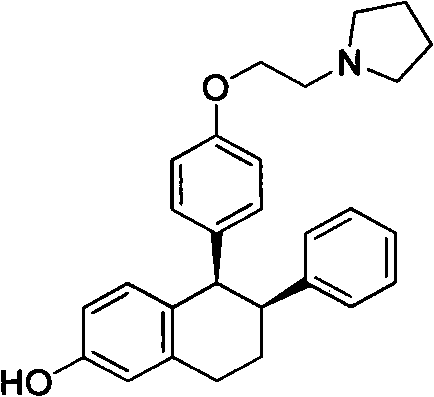
Method for preparing lasofoxifene intermediate
A lasoxifene and intermediate technology, applied in the field of chemistry, can solve the problems of low overall yield, high production cost, and high requirements for production equipment, and achieve the effects of cheap raw materials, improved overall yield, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 1-(2-(4-(6-methoxy-3,4-dihydro-1-yl)phenoxy)ethyl)pyrrolidine hydrochloride (compound II')
[0031]
[0032] Put 2.7g (114mmol) of magnesium ribbon in a 500mL three-necked flask, add 10mL of tetrahydrofuran, add a few drops of 1,2-dibromoethane while stirring, add dropwise 114mL of a tetrahydrofuran solution of 31g (114mmol) of compound I, and after the dropwise addition Start to heat the reaction to reflux until all the magnesium bands are dissolved and disappear, then add 20 g (114 mmol) of 6-methoxytellone dropwise in 114 mL of tetrahydrofuran solution, and continue the reflux reaction overnight after the addition is complete. After the reaction, cool to room temperature, add 50mL of water and 200mL of ethyl acetate, stir for 10 minutes, filter with diatomaceous earth, rinse with ethyl acetate several times, concentrate the filtrate, add 200mL of 2N hydrochloric acid to the residue, and wash the aqueous solution with methanol base tert-butyl ether, ...
Embodiment 2
[0035] Preparation of 1-(2-(4-(6-methoxy-3,4-dihydro-1-yl)phenoxy)ethyl)pyrrolidine (Compound II)
[0036]
[0037] 5g of compound II' (refer to Example 1 for the preparation method) was dissolved in 50mL of dichloromethane, washed with 25mL of saturated aqueous sodium carbonate solution, and then washed with 25ml of saturated aqueous sodium chloride solution, and the organic phase was dried over anhydrous sodium sulfate, concentrated, vacuum- After drying, compound II (4.5 g, yield 99%) was obtained. Compound II Identification Parameters:
[0038] (MS: 350.2[P + +1]); 1 H NMR (400MHz, CDCl 3 ): δ7.28(d, J=8.4Hz, 2H), 6.95(m, 3H), 6.79(d, J=2.6Hz, 1H), 6.65(dd, J=8.4, 2.6Hz, 1H), 5.93 (t, J=4.6Hz, 1H), 4.18(t, J=6.0Hz, 2H), 3.82(s, 3H), 2.98(m, 2H), 2.83(m, 2H), 2.69(m, 4H) , 2.39(m, 2H), 1.86(m, 4H).
Embodiment 3
[0040] Preparation of 1-(2-(4-(2-bromo-6-methoxy-3,4-dihydronaphthalen-1-yl)phenoxy)ethyl)pyrrolidine hydrochloride (compound III')
[0041]
[0042]Compound II' 9.0g (25mmol, or equivalent compound II) was dissolved in 250mL tetrahydrofuran, and 8.0g (25mmol) of pyridinium tribromide was added, and stirred at room temperature for 3 days. The reaction solution was concentrated to dryness on a rotary evaporator, 100 mL of methyl tert-butyl ether was added to the residue, and filtered. The resulting solid was dissolved in 200 mL of dichloromethane, washed twice with 100 mL of 0.5N hydrochloric acid, then dried over anhydrous magnesium sulfate, and filtered. , and concentrated to obtain 12.4 g of a brownish-red solid, namely compound III', which was directly used in the next reaction. Identification parameters of compound III':
[0043] (MS: 428.2 / 430.2[P + +1]); 1 H NMR (400MHz, CDCl 3 ): δ11.77(br, 1H), 7.18(d, J=8.4Hz, 2H), 6.97(d, J=8.4Hz, 2H), 6.72(d, 1H), 6.57(m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com