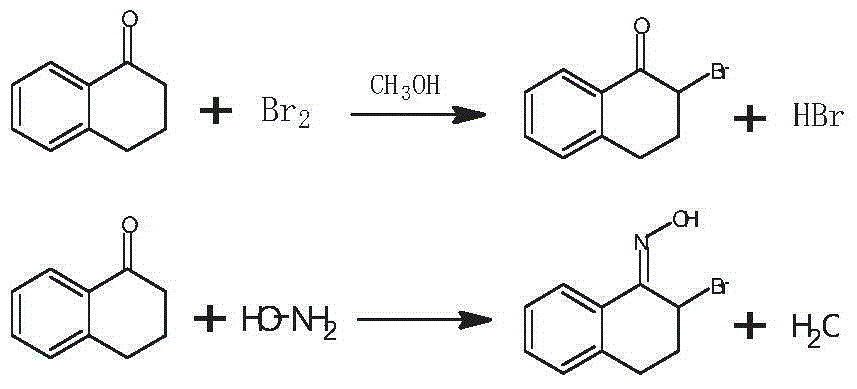
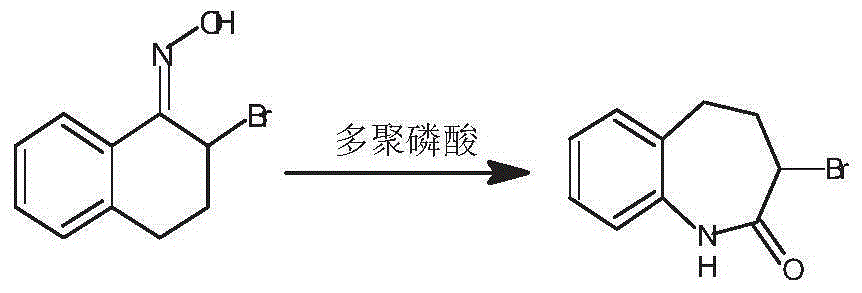
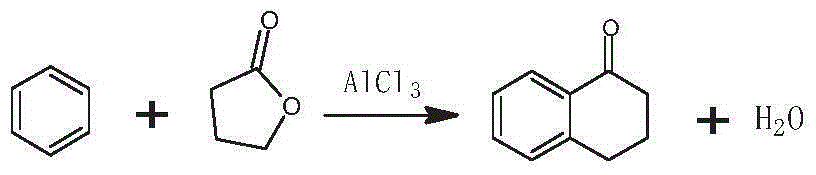Preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-keto
A technology of benzazepine and benzonitrogen, applied in the field of preparation of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one, which can solve the complicated purification , many by-products, low yield and other problems, to achieve the effect of simple production process, low production cost and high purity of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0035] The preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one in this embodiment includes the preparation stage of α-tetralone, 2-bromo-3 , 4-dihydro-N-hydroxy-(2H)-naphthylimine preparation stage and 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-one stage, Specific steps are as follows:
[0036] 1) α-tetralone preparation stage
[0037] Add γ-butyrolactone, benzene, and anhydrous aluminum trichloride in a molar ratio of 1:10:3 into the dry reaction kettle, slowly heat up to 65°C under stirring, and keep the temperature for 28 hours; 25 parts by weight of 6% hydrochloric acid solution are hydrolyzed, layered, and washed to neutrality; then excess benzene is removed by distillation, and high-vacuum rectification is used to obtain α-tetralone;
[0038] 2) 2-bromo-3,4-dihydro-N-hydroxy-(2H)-naphthalene imine preparation stage
[0039] Add α-tetralone and methanol in a molar ratio of 1:25 into the dry reactor, and under stirring, add bromine with a molar rat...
Embodiment 2
[0043] The preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one in this embodiment includes the preparation stage of α-tetralone, 2-bromo-3 , 4-dihydro-N-hydroxy-(2H)-naphthylimine preparation stage and 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-one stage, Specific steps are as follows:
[0044] 1) α-tetralone preparation stage
[0045] Add γ-butyrolactone, benzene, and anhydrous aluminum trichloride in a molar ratio of 2:17:6 into the dry reaction kettle, slowly heat up to 75°C under stirring, and keep the temperature for 20 hours; 25 parts by weight of 6% hydrochloric acid solution are hydrolyzed, layered, and washed to neutrality; then excess benzene is removed by distillation, and high-vacuum rectification is used to obtain α-tetralone;
[0046] 2) 2-bromo-3,4-dihydro-N-hydroxy-(2H)-naphthalene imine preparation stage
[0047] Add α-tetralone and methanol in a molar ratio of 1:50 into a dry reactor, and under stirring, add bromine dropwise at a temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com