Synthesis method of 2-chloro-4-phenylbenzoquinazoline
A benzoquinazoline and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, long reaction time, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
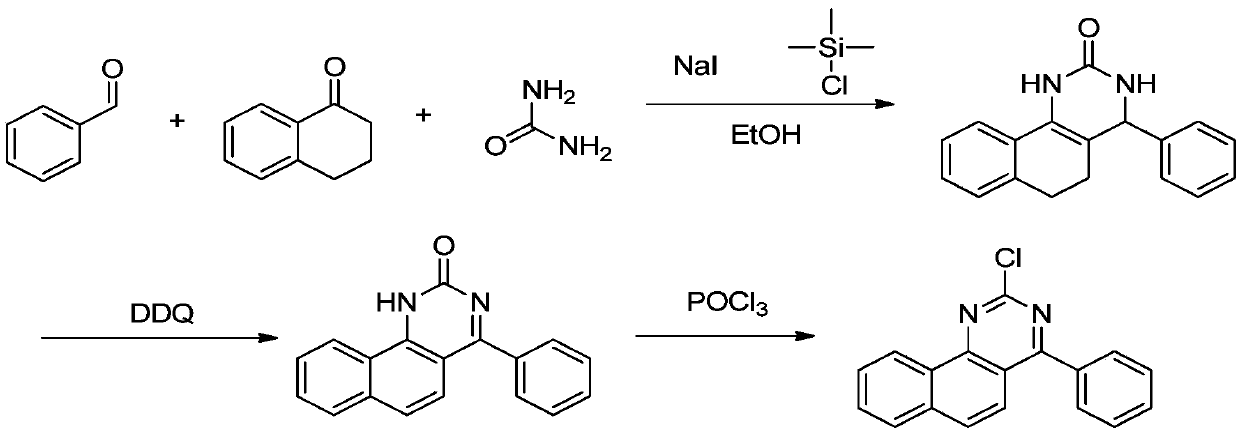
[0025] A kind of synthetic method of 2-chloro-4 phenyl benzoquinazoline, comprises the following steps:
[0026] The first step: 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one
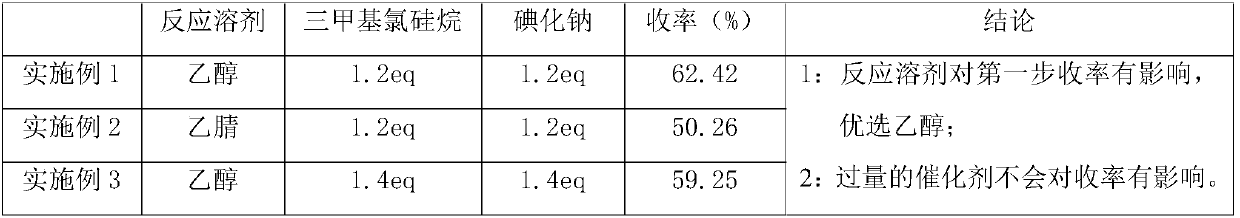
[0027] In a 500mL container, add 10.0g of 1-tetralone, 8.0g of benzaldehyde, 4.9g of urea, and 150ml of ethanol, add 8.9g of trimethylchlorosilane and 12.3g of sodium iodide under stirring, and heat up to 80°C for 2 hours. , cooled to room temperature and filtered after cooling down the reaction, and eluted the solid with a small amount of water and ethanol to obtain off-white solid 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one 11.8g , yield 62.42%;
[0028] The second step: Synthesis of 4-phenyl-1H-benzo[h]quinazolin-2-one:
[0029] In a 250mL container, add 11.8g of 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one synthesized in the first step above, 18.9g of DDQ, and 60ml of o-dichlorobenzene , raise the temperature to 70-75°C for 30 minutes, add 10.0g of DDQ, raise the temperature ...
Embodiment 2
[0033] A kind of synthetic method of 2-chloro-4 phenyl benzoquinazoline, comprises the following steps:
[0034] The first step: 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one
[0035] Add 10.0g of 1-tetralone, 8.0g of benzaldehyde, 4.9g of urea, 150ml of acetonitrile into a 500mL container, add 8.9g of trimethylchlorosilane and 12.3g of sodium iodide under stirring, and heat up to 80°C for 2 hours , cooled to room temperature and filtered after cooling down the reaction, and eluted the solid with a small amount of water and ethanol to obtain 9.5 g of off-white solid 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one , yield 50.26%;
[0036] The second step: Synthesis of 4-phenyl-1H-benzo[h]quinazolin-2-one:
[0037] In a 250mL container, add 11.8g of 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one synthesized in the first step above, 18.9g of DDQ, and 60ml of o-dichlorobenzene , raise the temperature to 70-75°C for 30 minutes, add 10.0g of DDQ, raise the temperat...
Embodiment 3
[0041] The first step: 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one
[0042]In a 500mL container, add 10.0g of 1-tetralone, 8.0g of benzaldehyde, 4.9g of urea, and 150ml of ethanol, add 10.4g of trimethylchlorosilane and 14.4g of sodium iodide under stirring, and heat up to 80°C for 2 hours , cooled to room temperature and filtered after cooling down the reaction, and eluted the solid with a small amount of water and ethanol to obtain 11.2 g of off-white solid 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one , yield 59.25%;
[0043] The second step: Synthesis of 4-phenyl-1H-benzo[h]quinazolin-2-one:
[0044] In a 250mL container, add 11.8g of 4-phenyl-3,4,5,6-tetrahydrobenzoquinazolin-2(1H)one synthesized in the first step above, 18.9g of DDQ, and 60ml of o-dichlorobenzene , raise the temperature to 70-75°C for 30 minutes, add 10.0g of DDQ, raise the temperature to 160-165°C for 2 hours, add 10ml of n-hexane to the system after the reaction is completely cooled dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com