Method for preparing agomelatine
A technology with structural formula and methoxy group, applied in the new field of agomelatine preparation, can solve the problems of low atom utilization rate, unfavorable environmental protection and high production cost, and achieves mild reaction, simple post-processing, and reaction yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
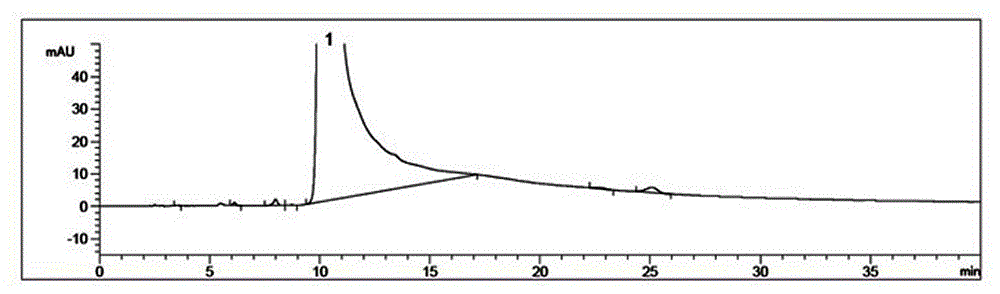
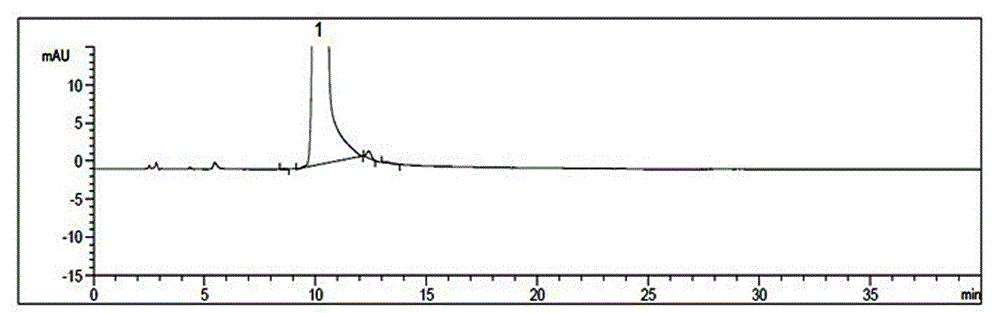
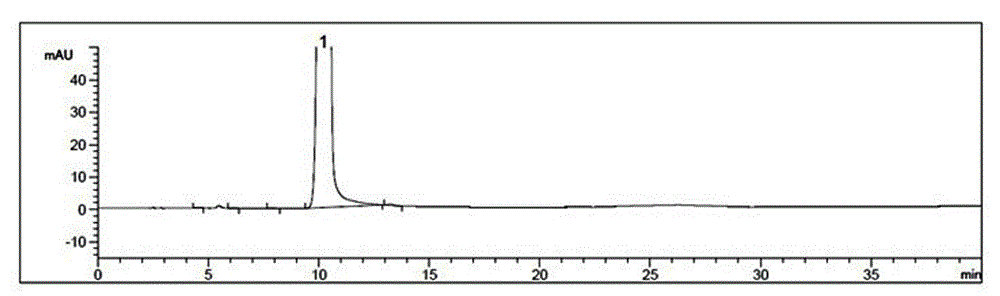
Image
Examples
Embodiment 1
[0049] Embodiment 1: agomelatine
[0050] Step 1: Preparation of (7-methoxy-3,4-dihydro-2H-naphthalene-1-ylidene)acetonitrile
[0051]Put 200ml of ethylene glycol dimethyl ether, 17.0 g (0.71mol) of sodium hydrogen into a 1000ml four-necked bottle, control the temperature at 15±2°C, add 50g (0.28mol) of 7-methoxy-1-tetralone, and stir For 30 minutes, 70.4 g (0.40 mol) of diethyl nitrile methyl phosphate was added dropwise to the reaction solution at 15 ° C, and the drop was completed in 3 to 4 hours. The temperature was controlled at 10 ° C, and the reaction was stirred for 15 hours. It was detected that the reaction was complete, and the reaction solution was cooled to Below 5°C, slowly add 200ml of water dropwise for 30 minutes, then adjust the pH to 5~6 with hydrochloric acid, add 2×100ml of dichloromethane for extraction twice, concentrate the organic layer to dryness under reduced pressure, add 200ml of 95% ethanol to the residue , heated up to dissolve, stirred for 1 ho...
Embodiment 2
[0058] Example 2 agolametine
[0059] Step 1: Preparation of (7-methoxy-3,4-dihydro-2H-naphthalene-1-ylidene)acetonitrile
[0060] 50g (0.28mol) of 7-methoxyl-1-tetralone and 70.4g (0.40mol) of diethyl nitrile methyl phosphate were reacted in N,N-dimethylformamide under sodium hydrogen catalysis, the Step all the other operations are with the step 1 of embodiment 1.
[0061] Step 2: Preparation of 1-cyano-7-methoxy-1-naphthylmethanol propionate
[0062] Put 185g (0.75mol) of chlorobenzoquinone and 600ml of propionic acid into the reaction bottle, stir, and add 30g of (7-methoxy-3,4-dihydro-2H-naphthalene-1-ylidene) acetonitrile obtained in step 1 (0.15mol), stirred for 1 hour, heated to reflux, and reacted for 12 hours. Cool to room temperature, distill the reaction solution under reduced pressure, recover acetic acid, add 200ml of toluene to the residue, stir for 1 hour, filter, wash the filtrate twice with 2×200ml of 5% sodium bisulfite aqueous solution, and then wash wi...
Embodiment 3
[0067] Example 3 Agomelatine
[0068] Step 1: Preparation of (7-methoxy-3,4-dihydro-2H-naphthalene-1-ylidene)acetonitrile
[0069] 50g (0.28mol) of 7-methoxy-1-tetralone and 70.4g (0.40mol) of diethyl nitrile methyl phosphate are reacted in dimethyl sulfoxide under sodium hydrogen catalysis, and the rest of the steps are the same as Step 1 of Example 1.
[0070] Step 2: Preparation of 1-cyano-1-(7-methoxy-1-naphthyl)methanol butyrate
[0071] Put 272g (1.2mol) of dichlorodicyanobenzoquinone and 700ml of n-butyric acid into the reaction bottle, stir, and add (7-methoxy-3,4-dihydro-2H-naphthalene-1- Base) 30 g (0.15 mol) of acetonitrile, stirred for 1 hour, heated to reflux, and reacted for 12 hours. Cool to room temperature, distill the reaction solution under reduced pressure, recover acetic acid, add 200ml of toluene to the residue, stir for 1 hour, filter, wash the filtrate twice with 2×200ml of 5% sodium bisulfite aqueous solution, and then wash with 2×100ml of Water, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com