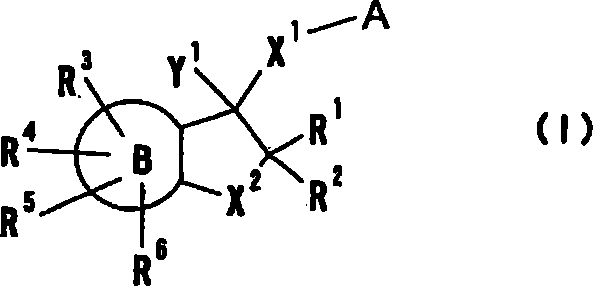
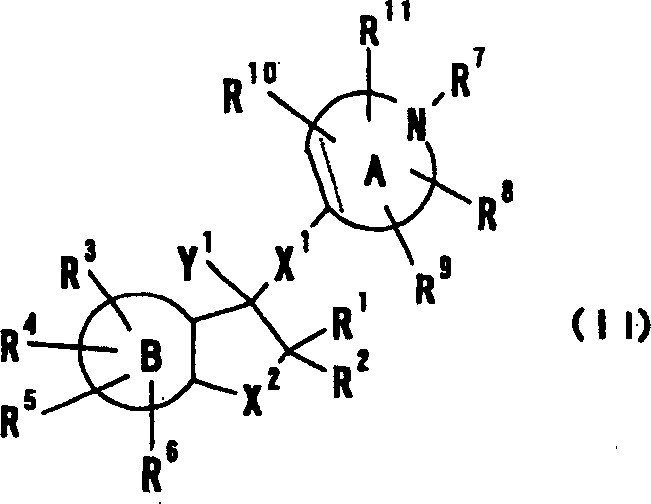
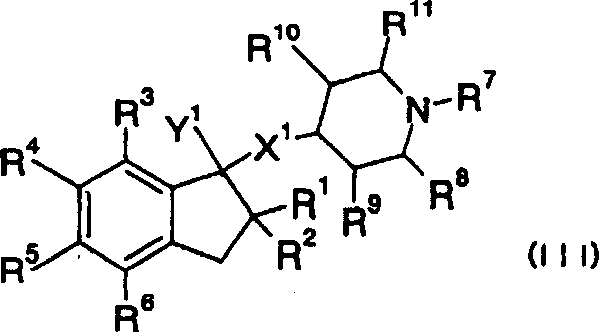
Cyclic amine derivative or salt thereof
A technology of cyclic amines and derivatives, which is applied in the field of NMDA receptor antagonists, and can solve the problems of unrecorded medical use of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0167] 1) Preparation of rat meninges specimens
[0168] After taking out the whole brains of 30 SD rats (available from Japan SLC Co., Ltd.) at 10 weeks after birth, the cerebellum was removed. A 0.32 M sucrose solution was added to the part containing the brain, and it was chopped with a blender, and then pulverized with a Teflon (registered trademark) homogenizer. Centrifuge at 2800 rpm at 4°C for 15 minutes, then centrifuge the supernatant at 15000 g at 4°C for 20 minutes. The resulting precipitate was suspended in 50 mM Tris-HCl (pH 7.5) containing 0.08% Triton X-100, allowed to stand on ice for 30 minutes, and then centrifuged at 15,000 g at 4° C. for 20 minutes. Add 50 mM Tris-HCl (pH 7.5) to the precipitate to suspend it, and then centrifuge at 15000 g at 4° C. for 20 minutes. After adding 50 mM Tris-HCl (pH 7.5) to the precipitate, centrifugation was performed in the same manner. 20 ml of 50 mM Tris-HCl (pH 7.5) was added to the precipitate to suspend it, and it wa...
Embodiment
[0187] Hereinafter, the compounds of the present invention will be described based on examples. In addition, novel compounds are also included in the raw material compounds of the compounds of the present invention, so these production examples are described below as reference examples.
reference example 1
[0189] 3-(3-Bromo-4-fluorophenyl)propanoic acid
[0190] Piperidine (1 ml) was added to a solution of 3-bromo-4-fluorobenzaldehyde (20 g) and malonic acid (51 g) in pyridine (250 ml), and the mixture was heated to reflux for 3 hours. After the reaction, the solvent was concentrated under reduced pressure, water was added, and 1N hydrochloric acid was added with stirring for neutralization. (2E)-3-(3-bromo-4-fluorophenyl)acrylic acid obtained by filtering the precipitate was dissolved in THF (200ml), and stirred at room temperature for 12 using 5% Rh-C (3g) under hydrogen atmosphere. Hour. After removing insoluble matter by filtration, the solvent was distilled off under reduced pressure to obtain the compound of Reference Example 1 (15 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com